Comparative Evaluation of Rapid Antigen Detection with Reverse Transcriptase Polymerase Chain Reaction for Detection of Novel SARS-CoV-2.
Abstract
Introduction: Swift and precise detection of SARS-CoV-2 is essential for managing outbreaks both within communities and hospitals. Real-time reverse transcriptase polymerase chain reaction (rRT–PCR) stands as the benchmark diagnostic test for SARS-CoV-2. However, its reliance on specialized equipment and technical expertise, alongside the necessity for a sophisticated laboratory, limits its widespread use. Rapid antigen tests have emerged as convenient point-of-care diagnostic assays. Evaluating the diagnostic accuracy of these tests compared to RT-PCR is crucial. While numerous studies have been conducted for this purpose globally, many have assessed performance using separate samples, potentially leading to variations in findings. Aim: In our study, we aimed to comparatively assess rRT-PCR and Rapid Antigen Tests, with rRT-PCR considered the gold standard, by conducting both tests using samples collected in the same Viral Transport Medium (VTM)
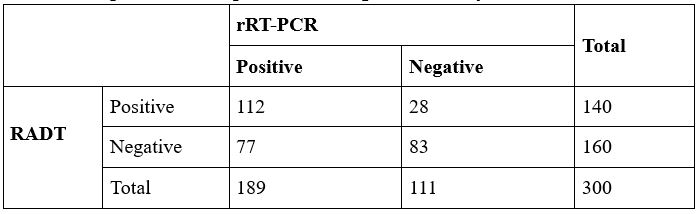
tube. Materials and Methods: We collected a total of 300 nasopharyngeal/oropharyngeal swabs from patients suspected of having COVID-19. Rapid antigen tests were performed directly from the tube using the STANDARD Q COVID-19 Ag test. RT-PCR of the sample was conducted post RNA extraction. Both tests were performed using the same VTM tube.Results: The rapid antigen detection test (RADT) demonstrated a sensitivity and specificity of 86% and 90%, respectively. The positive predictive value (PPV) and negative predictive value (NPV) of RADT were 91% and 88%, respectively. Conclusion: RADT conducted directly from VTM exhibited high sensitivity and specificity, suggesting its potential utility during pandemics.
Keywords: SARS CoV-2, RTPCR, RADT, Pandemic, Point of care
Downloads
References
2. WHO. Summary table of SARS cases by country, November 1, 2002-August 7, 2003. 2020. http://www.who.int/csr/sars/country/2003_08_15/en/
3. Zaki AM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367(19):1814-20.
4. World Health Organization. WHO coronavirus (COVID-19) dashboard 2022; (https:// covid19.who.int).
5. ICMR, Advisory on Strategy for COVID 19 Testing in India (Version 6), https://www.icmr.gov.in/cteststrat.html
6. The standard protocol for collection of nasal and oral swab samples as given by the WHO. 2020; https://www.who.int/csr/resources/publications/surveillance/CDS_EPR_ARO_2006_1.pdf
7. Porte L, Legarraga P, Vollrath V, Aguilera X, Munita JM, Araos R et al. Evaluation of a novel antigen based rapid detection test for the diagnosis of SARS CoV-2 in respiratory samples. International Journal of Infectious Diseases.2020; 99:328-33.
8. Halfon P, Penaranda G, Khiri H, Garcia V, Drouet H, Philibert P, et al. An optimized stepwise algorithm combining rapid antigen and RT-qPCR for screening of COVID-19 patients. PLoS ONE 2021; 16(9): e0257817.
9. Amadora PM,Donapetry PG, Fernandeze MD, Romoa FG, Castellano MAS, Estevez AS, et al. Clinitest rapid COVID-19 antigen test for the diagnosis of SARS CoV-2 infection: A multicenter evaluation study.Journal of Clinical Virology. 2021; 143:104961.
10. Peña M, Ampuero M, Garcés C, Gaggero A, García P, Velasquez MS, et al. Performance of SARS CoV-2 rapid antigen test compared with real-time RT-PCR in asymptomatic individuals. Int J Infect Dis. 2021;107:201-04.
11. Farhana A, Zahoor D, Wani S, Khan RA, Nasir R, Kanth F. Diagnostic utility and performance of rapid antigen test in SARS CoV- 2 in symptomatic and asymptomatic patients during the second pandemic wave in Kashmir, North India, Indian Journal of Medical Microbiology. 2022; https://doi.org/10.1016/ j.ijmmb.2022.06.007
12. Samantaray S, Nag VL, Rawat P, Misra S, Aggarwal A, Khan S et al. Demographic Profile of COVID-19 Cases: An Early Analysis of the Local Outbreak in a “Hotspot District” of Western Rajasthan in India. Asia Pacific Journal of Public Health. 2021; 33(1):138-40.
13. Chaimayo C, Kaewnaphan B, Tanlieng N, Athipanyasilp N, Sirijatuphat R, Chayakulkeeree M, et al. Rapid SARS CoV-2 antigen detection assayin comparison with real-time RT-PCR assayfor laboratory diagnosis of COVID-19 in Thailand. Virology Journal. 2020; 17:177
14. Soni SL, Kajal K, Yaddanapudi LN, Malhotra P, Puri GD, Bhalla A et al. Demographic & clinical profile of patients with COVID-19 at a tertiary care hospital in north India. Indian J Med Res.2021; 153:115-25.
15. Jegerlehner S, Riniker FS, Jent P, Bittel P, Nagler M. Diagnostic accuracy of a SARS CoV-2 rapid antigen test in real-life clinical settings. International Journal of Infectious Diseases. 2021; 109:118-22.
16. Mohan A, Tiwari P, Bhatnagar S, Patel A, Maurya A, Dar L et al. Clinico-demographic profile & hospital outcomes of COVID-19 patients admitted at a tertiary care centre in north India. Indian J Med Res. 2020; 152:61-69.
17. Jamil M, Bhattacharya P K, Barman B, et al. Clinical and Demographic Profile of COVID-19 Patients: A Tertiary Level Hospital-Based Study from Northeast India. Cureus. 2021; 13(10):e18881.
18. KritikosA, CaruanaG, Brouillet R, Miroz JP, Abed-MaillardS, StiegerG, et al. Sensitivity of Rapid Antigen Testing and RT-PCR Performed on Nasopharyngeal Swabs versus Saliva Samples in COVID-19 Hospitalized Patients: Results of a Prospective Comparative Trial (RESTART). Microorganisms 2021; 9:1910. https://doi.org/10.3390/ microorganisms9091910.
19. Lee J, Songb JU,Shim SR. Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS CoV-2 infection: A systematic review and meta-analysis. Journal of Clinical Virology. 2021; 144:104985.
20. Selvabai APR, Lino V. Koshy LV, Shanmugam P. Diagnostic Efficacy of COVID-19 Rapid Antigen Detection Card in Diagnosis of SARS CoV-2. J Lab Physicians 2022;14:324-28.
Copyright (c) 2024 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


