Comparison of various principles of coagulation tests in handling hemolysed blood samples
Abstract
Background: Rejection of hemolysed samples for coagulation test is the standard practice. However, when clinicians deal with extremely sick patients where repeat sampling is difficult to obtain, rejection of the sample is a lost opportunity for the lab physician to assist inpatient care. Proceeding with the test and providing a clinically helpful interpretation of the results will ensure the active participation of the laboratory physician. Different principles of coagulation testing handle the hemolysed samples differently. It is essential to know the best principle to proceed with the hemolysed sample if need be. This study set out to estimate the predictive values of post-hemolytic sample coagulation test results with various coagulation test principles.
Methods: This is a prospective experimental study where the non-hemolysed samples were processed for coagulation tests. Part of the sample was deliberately hemolysed, and the coagulation tests were repeated.
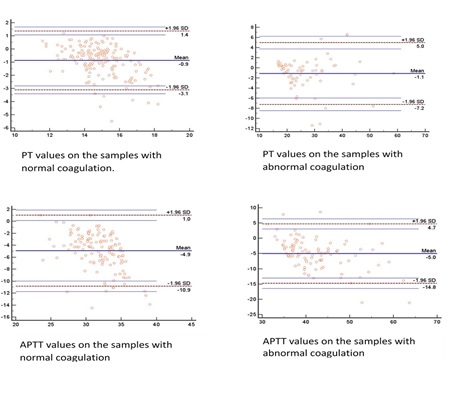
Results: Two hundred and forty-eight samples were studied. A median of 11% hemolysis was achieved experimentally. The mean difference in prothrombin time between pre and post hemolytic samples with normal PT was 0.9 and with abnormal PT, it was 1.1 seconds. The same for APTT was 4.9 and 1.1 seconds, respectively. The majority of the samples showed prolonged coagulation post hemolysis. Positive (PPV) and negative (NPV) predictive values for prothrombin time are 97.3 and 73.4%, respectively. Similarly, PPV and NPV for APTT are 97.4 and 47.1%, respectively.
Conclusions: Samples with normal values after hemolysis are more likely to be normal.
Downloads
References
Lippi G, Plebani M, Favaloro EJ. Interference in coagulation testing: focus on spurious hemolysis, icterus, and lipemia. Semin Thromb Hemost. 2013 Apr;39(3):258-66. doi: 10.1055/s-0032-1328972.
Carraro P, Servidio G, Plebani M. Hemolysed specimens: a reason for rejection or a clinical challenge? Clin Chem. 2000 Feb;46(2):306-7.
Heyer NJ, Derzon JH, Winges L, Shaw C, Mass D, Snyder SR. et al. Effectiveness of practices to reduce blood sample hemolysis in EDs: a laboratory medicine best practices systematic review and meta-analysis. Clin Biochem. 2012 Sep;45(13-14):1012-32. doi: 10.1016/j.clinbiochem.2012.08.002.
Lippi G, Plebani M, Di Somma S, Cervellin G. Hemolysed specimens: a major challenge for emergency departments and clinical laboratories. Crit Rev Clin Lab Sci. 2011 May-Jun;48(3):143-53. doi: 10.3109/10408363.2011.600228.
Plebani, Mario. "Errors in clinical laboratories or errors in laboratory medicine." Clinical Chemistry and Laboratory Medicine (CCLM) 44.6 (2006): 750-759.
Carraro P, Servidio G, Plebani M. Hemolysed specimens: a reason for rejection or a clinical challenge? Clin Chem. 2000 Feb;46(2):306-7.
Arkin, Charles F. Collection, Transport, and Processing of Blood Specimens for Testing Plasma Based Coagulation Assays: Approved Guideline. NCCLS, 2003.
Bodansky DMS, Lumley SE, Chakraborty R, Mani D, Hodson J, Hallissey MT, Tucker ON. Potential cost savings by minimization of blood sample delays on care decision making in urgent care services. Ann Med Surg (Lond). 2017 Jun 16;20:37-40. doi: 10.1016/j.amsu.2017.06.016.
Arora S, Kolte S, Dhupia J. Hemolysed Samples Should be Processed for Coagulation Studies: The Study of Hemolysis Effects on Coagulation Parameters. Ann Med Health Sci Res. 2014 Mar;4(2):233-7. doi: 10.4103/2141-9248.129049.
Laga AC, Cheves TA, Sweeney JD. The effect of specimen hemolysis on coagulation test results. Am J Clin Pathol. 2006 Nov;126(5):748-55. doi: 10.1309/03FK-3378-YTRA-1FRF.
Lippi G, Montagnana M, Salvagno GL, Guidi GC. Interference of blood cell lysis on routine coagulation testing. Arch Pathol Lab Med. 2006 Feb;130(2):181-4. doi: 10.5858/2006-130-181-IOBCLO.
Copyright (c) 2021 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


