A comparative study of conventional cytology and cell block method with immunohistochemistry in the diagnosis of serous effusions
Abstract
Introduction: Conventional smear (CS) examination of serous effusions is of paramount importance for diagnosis, staging, prognostication, and management of malignancy. The method has some disadvantages which can be overcome by cell block (CB) preparation. CB technique increases the diagnostic accuracy due to increased cellularity, preservation of tissue architecture and feasibility of performing immunohistochemistry (IHC).
Aims and Objectives: To assess and compare the diagnostic yields of CS and CB techniques for detection of malignancy in pleural and peritoneal effusions and to study the utility of CB preparation with special emphasis on the feasibility of performing IHC in identifying the primary site of malignancy in the cases of carcinoma of unknown primary (CUP).
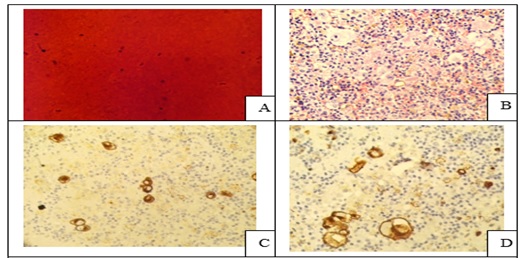
Materials and Methods: In ESI-PGIMSR, Manicktala, each of 104 fluid samples were divided into two equal parts: one part was subjected to CS technique, the smears were stained with Leishman-Giemsa and Papanicolaou stains while the other part was subjected to Plasma thromboplastin CB technique and the sections stained by Hematoxylin and Eosin. IHC was performed whenever required. Provisional diagnoses made on CSs were compared with the diagnoses revised after examining CB slides.
Results: Out of 104 fluid samples, on CS, 19 (18.27%) cases were positive for malignancy, whereas on CB 39(37.5%) cases were diagnosed as malignancy. The additional yield of malignancy was 19.23% more by the CB method. IHC done on CBs could suggest the possible primary site in 31 cases.
Conclusions: The current study reports the diagnostic efficiency of the CB method to be superior to that of conventional smear alone as it has various advantages. Hence CB preparation should be routinely incorporated along with the use of IHC, if required, in the evaluation of serous effusions for a more accurate diagnosis.
Downloads
References
Dekker A, Bupp P. Cytology of Serous Effusions: An Investigation into the Usefulness of Cell Blocks versus Smears. Am J Clin Pathol. 1978;70(6):855-860. doi: https://doi.org/10.1093/ajcp/70.6.855.
Mishra R, Sharma A, Goyal V, Goyal V, Thapar M. Critical analysis of cell block versus smear examination in effusions. J Cytol. 2009;26(2):60-64. doi: https://dx.doi.org/10.4103%2F0970-9371.55223.
Mezger J, Stotzer O, Schilli G, Bauer S, Wilmanns W. Identification of carcinoma cells in ascitic and pleural fluids. Comparison of four panepithelial antigens with the carcinoembryonic antigen. Acta Cytologica. 1992;36(1):75-81.
Price B, Ehya H, Lee J. Significance of pericellular lacunae in cell blocks of effusions. Acta Cytologica. 1992;36(3):333-337.
Grandhi B, Shanthi V, Rao NM, Reddy VC, Mohan K. The diagnostic utility of cell block as an adjunct to cytological smears. Int J Med Res Health Sci. 2014;3(2):278-284.
Kung I, Yuen R, Chan J. Optimal formalin fixation and processing schedule of cell blocks from fine needle aspirates. Pathology. 1989;21(2):143-145. doi: https://doi.org/10.3109/00313028909059552.
Zito F, Gadaleta C, Salvatore C, Filático R, Labriola A, Marzullo A et al. A modified cell block technique for fine needle aspiration cytology. Acta Cytologica. 1995;39(1):93-99.
Leung S, Bedard Y. Simple miniblock technique for cytology. Modern Patholology. 1993;6(5):630-632.
Kushwaha R, Shashikala P, Hiremath S, Basavaraj H. Cells in pleural fluid and their value in differential diagnosis. J Cytol. 2008;25(4):138.
Krogerus L, Andersson L. A simple method for the preparation of paraffin-embedded cell blocks from fine needle aspirates, effusions and brushings. Acta Cytologica. 1998;32(4):585-587.
Bahrenburg L. On the diagnostic results of the microscopical examination of the ascitic fluid in two cases of carcinoma involving the peritoneum. Cleveland Medical Gazette.1896; 11:274-278.
Shivakumarswamy U, Karigowdar M, Arakeri S, Yelikar B. Diagnostic utility of the cell block method versus the conventional smear study in pleural fluid cytology. J Cytol. 2012;29(1):11.
Bancroft J, Gamble M. Theory and practice of histological techniques. Philadelphia: Churchill Livingstone Elsevier; 2011.
Yang G, Wan L, Papellas J, Waisman J. Compact Cell Blocks. Acta Cytologica. 1998;42(3):703-706. doi: https://doi.org/10.1159/000331830.
Esteban J, Yokota S, Husain S, Battifora H. Immunocytochemical Profile of Benign and Carcinomatous Effusions: A Practical Approach to Difficult Diagnosis. Am J Clinic Pathol. 1990;94(6):698-705. doi: https://doi.org/10.1093/ajcp/94.6.698.
Cibas E, Corson J, Pinkus G. The distinction of adenocarcinoma from malignant mesothelioma in cell blocks of effusions: The role of routine mucin histochemistry and immunohistochemical assessment of carcinoembryonic antigen, keratin proteins, epithelial membrane antigen, and milk fat globule-derived antigen. Human Pathology. 1987;18(1):67-74. doi: https://doi.org/10.1016/s0046-8177(87)80196-x.
Mason MR, Bedrossian CW, and Fahey CA. Value of immunocytochemistry in the study of malignant effusions. Diagn Cytopathol.1987;3(3):215-221. doi: https://doi.org/10.1002/dc.2840030308.
Nance K, Silverman J. Immunocytochemical Panel for the Identification of Malignant Cells in Serous Effusions. Am J Clin Pathol. 1991;95(6):867-874. doi: https://doi.org/10.1093/ajcp/95.6.867.
Gray W, Kocjan G. Diagnostic cytopathology. [Edinburgh]: Churchill Livingstone/Elsevier; 2010.
Doglioni C, Dei A, Laurino L, Iuzzolino P, Chiarelli C, Celio M et al. Calretinin: A Novel Immunocytochemical Marker for Mesothelioma. Am J Surg Pathol. 1996;20(9):1037-1046. doi: https://doi.org/10.1097/00000478-199609000-00001.
Barberis M, Faleri M, Veronese S, Casadio C, Viale G. Calretinin. Acta Cytologica. 1997;41(6):1757-1761. doi: https://doi.org/10.1159/000333181.
Ko E, Jhala N, Shultz J, Chhieng D. Use of a Panel of Markers in the Differential Diagnosis of Adenocarcinoma and Reactive Mesothelial Cells in Fluid Cytology. Am J Clin Pathol. 2001;116(5):709-715. doi: https://doi.org/10.1309/pj7h-a52v-m3xb-v94y.
Ghosh I, Dey S, Das A, Bhattacharjee D, Gangopadhyay S, Cell block cytology in pleural effusion. J Indian Med Assoc. 2012; 110(6):390-392.
Kulkarni M, Desai S, Ajit D, Chinoy R. Utility of the thromboplastin-plasma cell-block technique for fine-needle aspiration and serous effusions. Diagnos Cytopathol. 2009;37(2):86-90. doi: https://doi.org/10.1002/dc.20963.
Keyhani-Rofagha S, Vesey-Shecket M. Diagnostic value, feasibility, and validity of preparing cell blocks from fluid-based gynecologic cytology specimens. Cancer. 2002;96(4):204-209. doi: https://doi.org/10.1002/cncr.10716.
Santwani P, Vachhani J. Analysis of Diagnostic Value of Cytological Smear Method Versus Cell Blocks Method in Body Fluid Cytology: Study of 150 Cases. Ethiop J Health Sci. 2014;24(2):125-131. https://dx.doi.org/10.4314%2Fejhs.v24i2.4.
Bansode S, Kumbalkar D, Nayak S. Evaluation of Cell Block Technique in the Cytodiagnosis of Body Fluids. Int J Sci Res. 2015;4(7):87-94.
Padmavathi A, B.V S, B A. A comparative study of fluid cytology with smear and cell block preparation. J Evid Based Med Healthc. 2016;3(65):3532-3535. doi: https://doi.org/10.18410/jebmh/2016/758.
Khan N, Sherwani R, Afroz N, Kapoor S. Cytodiagnosis of malignant effusion and determination of primary site. J Cytol. 2005;22(3):107‑108.
Fagere M. Diagnostic Utility of AgNORs Staining of Serous Effusion among Sudanese Patients. Int J Sci Tech. 2016;5(1):36-42
Richardson H, Koss L, Simon T. An evaluation of the concomitant use of cytological and histocytological techniques in the recognition of cancer in exfoliated material from various sources. Cancer. 1955;8(5):948-950. doi: https://doi.org/10.1002/1097-0142(1955)8:5%3C948::aid-cncr2820080515%3E3.0.co;2-m.
Liu K, Dodge R, Glasgow B, Layfield L. Fine-needle aspiration: Comparison of smear, cytospin, and cell block preparations in diagnostic and cost effectiveness. Diagnostic Cytopathol. 1998;19(1):70-74. doi: https://doi.org/10.1002/(sici)1097-0339(199807)19:1%3C70::aid-dc15%3E3.0.co;2-5.
Shukla P, Kaur S, Gulwani H. Diagnostic utility of plasma thromboplastin cell block preparation in cytological evaluation of serous effusions. Int J Biomed Res. 2015;6(11):890-896.
Shivakumarswamy U, Arakeril S, Karigowdar M, Yelikar B. The role of the cell block method in the diagnosis of malignant ascitic fluid effusions. J Clin Diagnos Res. 2012;6:1280–1283.
Bodele A, Parate S, Wadadekar A, Bobhate S, Munshi M. Diagnostic utility of cell block preparation in reporting of fluid cytology. J Cytol. 2003;20(3):133-135.
Poorana P. Cytological analysis of body fluids in conventional smear and cell block technique – Study of 120 cases. Int J Pharma Bio Sci. 2015;6(4):609-615.
Sujathan K, Kannan S, Mathew A, Pillai K, Chandralekha B, Nair M. Cyto-diagnosis of serous effusions: A combined approach to morphological features in Papanicolaou and May-Grunwald Giemsa stained smears and a modified cell block technique. J Cytol. 2000;17(2):89-95.
Nathani R, Hazari R, Patle Y, Gupta S. Comparative analysis of cavity effusions by cell blocks and smear examination. Int J Rec Trend Sci Technol. 2014;12(1):69-72.
Pomjanski N, Juergen GH, Doganay P, Schmiemann V, Buckstegge B, Böcking, A. Immunocytochemical identification of carcinomas of unknown primary in serous effusions. Diagnos Cytopathol. 2005;33(5):309-315. doi: https://doi.org/10.1002/dc.20393.
Nair G, Manjula A. Comparative study of cell blocks and routine cytological smears of pleural and peritoneal fluids in suspected cases of malignancy. Indian J Pathol Oncol. 2015;2(2):61-68.
Gaur D, Chauhan N, Kusum A, Harsh M, Talekar M, Kishore S et al. Pleural fluid analysis - role in diagnosing pleural malignancy. J Cytol. 2007;24(4):183.
Sears D, Hajdu S. The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytologica. 1987;31(2):85-97.
Murphy W, Ng A. Determination of Primary Site by Examination of Cancer Cells in Body Fluids. Am J Clin Pathol. 1972;58(5):479-488. doi: https://doi.org/10.1093/ajcp/58.5.479.
Monte S, Ehya H, Lang W. Positive effusion cytology as the initial presentation of malignancy. Acta Cytologica. 1987;31(4):448-452.
Foot N. Identification of types and primary sites of metastatic tumors from exfoliated cells in serous fluids. Am J Pathol. 1954;30(4):661-677.
Foot N. The identification of neoplastic cells in serous effusions; critical analysis of smears from 2,029 persons. Am J Pathol. 1956;32(5):961-977.
Fred J, Molengraft V, Peter V. The interval between the diagnosis of malignancy and the development of effusions, with reference to the role of cytologic diagnosis. Acta Cytologica 1988;32(2):183-187.
Bonito L, Falconieri G, Colautti I, Bonifacio D, Dudine S. The positive pleural effusion. A retrospective study of the cytopathologic diagnosis along with the autopsy conformation. Acta Cytologica. 1992;36(3):329-332.
Karki S, Jha A, Sayami G. The Role of Argyrophilic Nucleolar Organizer Region (AgNOR) Study in Cytological Evaluation of Fluids, Especially for Detection of Malignancy. Kathmandu Univ Med J. 2012;10(1):34-39. doi: https://doi.org/10.3126/kumj.v10i1.6913.
Risberg B. Flow cytometric immunophenotyping of serous effusions and peritoneal washings: comparison with immunocytochemistry and morphological findings. J Clin Pathol. 2000;53(7):513-517. doi: https://dx.doi.org/10.1136%2Fjcp.53.7.513.
Spieler P, Gloor F. Identification of types and primary sites of malignant tumors by examination of exfoliated tumor cells in serous fluids. Comparison with the diagnostic accuracy on small histologic biopsies. Acta Cytologica. 1985;29(5):753-767.
Copyright (c) 2020 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


