Expression of EGFR in non small cell lung carcinoma
Abstract
Background:The incidence and mortality associated with lung cancers are increasing at an alarming rate. Studies have shown that activation of Epidermal Growth Factor Receptor (EGFR) triggers the tumorigenesis in these cancers. The potential role of analyzing EGFR expression in these carcinomas could go a long way in devising screening tools for early detection of lung carcinoma. This study was carried out to evaluate the prevalence and factors associated with EGFR expression.
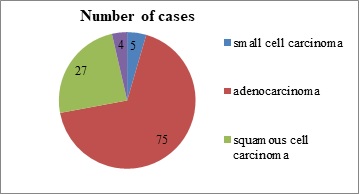
Methods: This cross sectional study was carried out among 75 paraffin block specimens of lung carcinoma received in our tertiary care center for a period of five years. Three micron thick paraffin sections were cut and Hemotoxylin & Eosin staining was done. All the adenocarcinoma cases were immunostained with EGFR antibody and the results were analyzed.
Results: Majority of the tumors were moderately differentiated (46%) and were negative for lymph node metastasis (69%). With regards to EGFR positivity, majority of the tumors showed EGFR expression 3+ (57.3%) flowed by 2+ (16%). Among the EGFR negative cases, ALK expression was positive in 5% of the cases.
Conclusion: The present study has reinforced the fact that Indian patients have high expression of EGFR and therefore can be benefitted by targeted therapy.IHC coupled with molecular analysis would be of maximum benefit to patients with EGFR & ALK mutations.
Downloads
References
2. Noronha V, Pinninti R, Patil VM, Joshi A, Prabhash K. Lung cancer in the Indian subcontinent. South Asian J Cancer. 2016;5(3):95-103. doi: 10.4103/2278-330X.187571.
3. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-94. doi: 10.4065/83.5.584.
4. de Mello RA, Marques DS, Medeiros R, Araújo AM. Epidermal growth factor receptor and K-Ras in non-small cell lung cancer-molecular pathways involved and targeted therapies. World J Clin Oncol. 2011;2(11):367-76. doi: 10.5306/wjco.v2.i11.367.
5. Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786-92. DOI:10.1056/NEJMoa044238
6. Dacic S. Molecular diagnostics of lung carcinomas. Arch Pathol Lab Med. 2011;135(5):622-9. doi: 10.1043/2010-0625-RAIR.1.
7. Brevet M, Arcila M, Ladanyi M. Assessment of EGFR mutation status in lung adenocarcinoma by immunohistochemistry using antibodies specific to the two major forms of mutant EGFR. J Mol Diagn. 2010;12(2):169-76. doi: 10.2353/jmoldx.2010.090140. Epub 2010 .
8. Bonanno L, Favaretto A, Rugge M, Taron M, Rosell R. Role of genotyping in non-small cell lung cancer treatment: current status. Drugs. 2011;71(17):2231-46. doi: 10.2165/11597700-000000000-00000.
9. Sahoo R, Harini VV, Babu VC, Patil Okaly GV, Rao S, Nargund A, et al. Screening for EGFR mutations in lung cancer, a report from India. Lung Cancer. 2011;73(3):316-9. doi: 10.1016/j.lungcan.2011.01.004. Epub 2011 Feb 18.
10. Engel KB, Moore HM. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2011;135(5):537-43. doi: 10.1043/2010-0702-RAIR.1.
11. Wen YH, Brogi E, Hasanovic A, Ladanyi M, Soslow RA, Chitale D, et al. Immunohistochemical staining with EGFR mutation-specific antibodies: high specificity as a diagnostic marker for lung adenocarcinoma. Mod Pathol. 2013;26(9):1197-203. doi:
10.1038/modpathol.2013.53. Epub 2013 Apr 19.
12. Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18(4):904-14. doi:10.1200/JCO.2000.18.4.904
13. Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201-8. Epub 2004 . doi:10.1200/JCO.2004.10.182
14. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337-45. doi:10.1056/NEJMoa033025
15. Pérez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, et al. Determinants of tumor response and survival with erlotinib in patients with non--small-cell lung cancer. J Clin Oncol. 2004;22(16):3238-47. doi:10.1200/JCO.2004.11.057
16. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290(16):2149-58. doi:10.1001/jama.290.16.2149
17. Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-8.doi: 10.1158/1078-0432.CCR-03-0564
18. Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R. FDA drug approval summary: erlotinib (Tarceva) tablets. Oncol. 2005;10(7):461-6. doi:10.1634/theoncologist.10-7-461
19. Sahoo R, Harini VV, Babu VC, Patil Okaly GV, Rao S, Nargund A, et al. Screening for EGFR mutations in lung cancer, a report from India. Lung Cancer. 2011;73(3):316-9. doi: 10.1016/j.lungcan.2011.01.004. Epub 2011.
20. Liang Z, Zhang J, Zeng X, Gao J, Wu S, Liu T. Relationship between EGFR expression, copy number and mutation in lung adenocarcinomas. BMC Cancer. 2010;10:376. doi: 10.1186/1471-2407-10-376.
21. Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15(18):5626-45. doi: 10.1158/1078-0432.CCR-09-0376.
22. Wang F, Wang S, Wang Z, Duan J, An T, Zhao J, et al. Phosphorylated EGFR expression may predict outcome of EGFR-TKIs therapy for the advanced NSCLC patients with wild-type EGFR. J Exp Clin Cancer Res. 2012;31(1):65. doi: 10.1186/1756-9966-31-65.
23. Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895-902. doi: 10.1200/JCO.2011.40.1174. Epub 2013 .
24. Rosell R, Moran T, Cardenal F, Porta R, Viteri S, Molina MA, et al. Predictive biomarkers in the management of EGFR mutant lung cancer. Ann N Y Acad Sci. 2010;1210:45-52. doi: 10.1111/j.1749-6632.2010.05775.x.
25. Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21(20):3798-3807. doi: 10.1200/JCO.2003.11.069Epub 2003 .
26. Rüschoff J, Kerr KM, Grote HJ, Middel P, von Heydebreck A, Alves VA, et al. Reproducibility of immunohistochemical scoring for epidermal growth factor receptor expression in non-small cell lung cancer: round robin test. Arch Pathol Lab Med. 2013;137(9):1255-61. doi: 10.5858/arpa.2012-0605-OA. Epub 2012 Dec 27.
27. Wang F, Wang J, Bai H, Zhao J, Wang Z, Zhuot M et al. An evaluation of phosphorylated EGFR expressionin predicting outcome of EGFR-TKI therapy for the advanced NSCLC patients with EGFR wild type. J ClinOncol. 2011;29(15 Suppl):7532. doi: 10.1200/jco.2011.29.15_suppl.7532
28. Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013 ;31(7):895-902. doi: 10.1200/JCO.2011.40.1174. Epub 2013 .
29. Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-59. doi: 10.1097/JTO.0b013e318290868f.
30. Rüschoff J, Kerr KM, Grote HJ, Middel P, von Heydebreck A, Alves VA, et al. Reproducibility of immunohistochemical scoring for epidermal growth factor receptor expression in non-small cell lung cancer: round robin test. Arch Pathol Lab Med. 2013;137(9):1255-61. doi: 10.5858/arpa.2012-0605-OA. Epub 2012 Dec 27.
31. Zhang X, Zhang S, Yang X, Yang J, Zhou Q, Yin L, et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010;9:188. doi: 10.1186/1476-4598-9-188.
32. Takeuchi K, Choi YL, Soda M, Inamura K, Togashi Y, Hatano S, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14(20):6618-24. doi: 10.1158/1078-0432.CCR-08-1018.
33. Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115(8):1723-33. doi: 10.1002/cncr.24181.
34. Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275-83. doi: 10.1158/1078-0432.CCR-08-0168.
35. Hofman P, Ilie M, Hofman V, Roux S, Valent A, Bernheim A, et al. Immunohistochemistry to identify EGFR mutations or ALK rearrangements in patients with lung adenocarcinoma. Ann Oncol. 2012;23(7):1738-43. doi: 10.1093/annonc/mdr535. Epub 2011 Nov 18.
36. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-703. doi: 10.1056/ NEJMoa1006448.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


