HER2/neu status detection in breast carcinoma: Is FISH the preferred approach over IHC
Abstract
Background: HER2/neu amplification is found in 15-30% of all breast cancer patients, which can be detected by either immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). The concordance of FISHand IHC analysis for HER2/neu amplification remains limited, especially with studies in the Indian population. There is a need to further classify this information as HER2/neu positive patients often have a worse disease prognosis and require anti-HER2/neu therapy.
Methods: In aretrospective study 149 patients with invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC), who underwent bothIHC and FISH testing for HER2/neu amplification were analysed to determine the concordance between the two tests in this population.
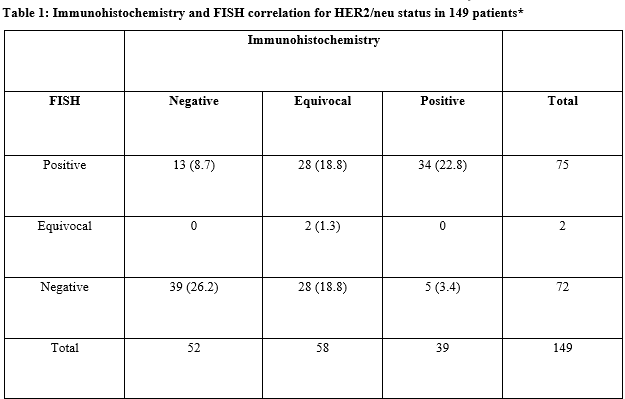
Results: Out of 149 patients reviewed, 58 had equivocal results on IHC, 52 patients had negative results and 39 patients had positive results on IHC. Analysis of the 91 non-equivocal IHC cases and their FISH results demonstrated an inter-rater reliability of Kappa= 0.606 (p <0.0005) 95% CI (0.445, 0.767). Of the 52 patients with negative IHC scores, 13 (25%) were found to be positive on FISH testing for HER/neu amplification. This represents a substantial number of patients who otherwise would not have received anti-HER2/neu therapy.
Conclusions: The present results indicate that FISH testing for HER2/neu status should be done on all breast cancer patients whenever possible, irrespective of IHC score status so that appropriate treatment decisions can be made. The higher sensitivity and specificity ofFISH testing can reduce the number of both false positive and false negatives seen with immunohistochemistry testing in the present study.
Downloads
References
Fornier M, Risio M, Van Poznak C, Seidman A. HER2 testing and correlation with efficacy of trastuzumab therapy. Oncology (Williston Park). 2002;16(10):1340-51.
Nandakumar A, Ramnath T, Chaturvedi M. The magnitude of cancer breast in India: a summary. Indian J Surg Oncol. 2010;1(1):8-9.doi: 10.1007/s13193-010-0004-z
Bethune GC, Pettit AS, Veldhuijzen van Zanten D, Barnes PJ. Well‐differentiated invasive breast cancers with equivocal HER 2 immunohistochemistry: what is the yield of routine reflex in‐situ hybridization testing? Histopathol. 2017;70(6):966-974.doi: 10.1111/his.13160.
Brunelli M, Manfrin E, Bria E, Massari F, Tortora G, Brunello E et al. HER2/neu gene determination in women screened for breast carcinoma: how screening programs reduce the skyrocketing cost of targeted therapy. Antican Res.2013;33(9):3705-10.
Varga Z, Noske A. Impact of modified 2013 ASCO/CAP guidelines on HER2 testing in breast cancer. One year experience. PLoSOne 2015;10(10):e0140652. doi: 10.1371/journal.pone.0140652.
Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. MolBiolInt 2014;2014:852748. doi: 10.1155/2014/852748.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J ClinOncol. 2013;31(31):3997-4013.doi: 10.1200/JCO.2013.50.9984
Kakar S, Puangsuvan N, Stevens JM, Serenas R, Mangan G, Sahai S, et al. HER-2/neu assessment in breast cancer by immunohistochemistry and fluorescence in situ hybridization: comparison of results and correlation with survival. MolDiagn. 2000;5(3):199-207.doi: 10.1054/modi.2000.16690
Tubbs RR, Pettay JD, Roche PC, Stoler MH, Jenkins RB, Grogan TM. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: apparent immunohistochemical false-positives do not get the message. J Clin Oncol 2001;19(10):2714-21. doi:10.1200/JCO.2001.19.10.2714.
Gokhale S, Gatalica Z, Mohammad A, Rampy AI, VelagaletiGopalrao VN. FISH for HER-2/neu in breast cancer: Standardization makes the difference! Indian J Cancer. 2004;41(4):152–158.
Dybdal N, Leiberman G, Anderson S, McCune B, Bajamonde A, Cohen RL, et al. Determination of HER2 gene amplification by fluorescence in situ hybridization and concordance with the clinical trials immunohistochemical assay in women with metastatic breast cancer evaluated for treatment with trastuzumab. Breast Cancer Res Treat. 2005;93(1):3–11. doi.org/10.1007/s10549-004-6275-8.
Lebeau A, Deimling D, Kaltz C, Sendelhofert A, Iff A, Luthardt B, et al. HER-2/neu analysis in archival tissue samples of human breast cancer: Comparison of immunohistochemistry and fluorescence in situ hybridization. J ClinOncol. 2001;19(2):354–363. doi: 10.1200/JCO.2001.19.2.354.
Wu SF, Liu YY, Liu XD, Jiang Y, Luo YF, Cui QC et al. HER2 gene status and mRNA expression in immunohistochemistry 1+ breast cancer. Chin J Pathol. 2018;47(7):522-526.doi: 10.3760/cma.j.issn.0529-5807.2018.07.008.
Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J ClinOncol. 2018;36(20):2105-2122.doi: 10.1200/JCO.2018.77.8738.
Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R,et al.Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J ClinOncol. 2000;18(21):3651-64. doi:10.1200/JCO.2000.18.21.3651
Solomon JP, Dell’Aquila M, Fadare O, Hasteh F. Her2/neu status determination in breast cancer: a single institutional experience using a dual-testing approach with immunohistochemistry and fluorescence in situ hybridization. Am J ClinPathol. 2017;147(4):432-437.doi: 10.1093/ajcp/aqw224.
KalalIravathyGoud SD, Vijayalaxmi K, Babu SJ, Vijay AR. Evaluation of HER-2/neu status in breast cancer specimens using immunohistochemistry (IHC) & fluorescence in-situ hybridization (FISH) assay. Indian J Med Res.2012;135(3):312-7.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


