Detection of extended-spectrum beta-lactamases in Pseudomonas aeruginosa and Acinetobacter baumanniiand their prevalence in Intensive care unit of a tertiary care hospital
Abstract
Introduction: Pseudomonas aeruginosa and Acinetobacter baumannii have been known to cause variety of infections, among patients admitted in Intensive Care Unit.Non fermenting Gram‑negative bacilli are developing resistance to commonly used antibiotics therefore are becoming difficult to treat Among various enzymes produced by bacteria which lead to drug resistance, extended‑spectrumbeta‑lactamase (ESBL) enzymesis one of the important mechanism of drug resistance. Thisstudy that was conducted a) To detect multidrug-resistant P. aeruginosa and A. baumanniiin patients admitted in ICU patients. b) To determine the prevalence of ESBL producing clinical isolates of Pseudomonas aeruginosa and Acinetobacter sp. in the ICU of the tertiary care hospital.
Material and Methods: The study was performed in the microbiology department of a North Indian rural tertiary care hospital (Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar, India) over a period of one year (January2012 to December 2012). The study included 100 isolates each of Acinetobacter baumannii & P. aeruginosa. Identification of both organisms was done using the standard microbiological techniques as described by Colle et al 1996.The antimicrobial susceptibility testing was performed by Kirby Bauer disc diffusion method. To detect ESBL producing isolates phenotypicaly, Disc approximation test was performed.
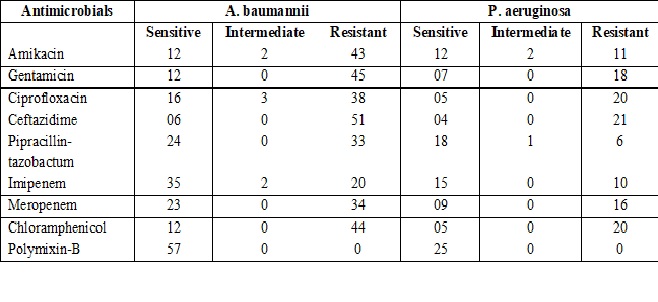
Results: Out of 200 isolates, 100 each of A. baumannii and P. aeruginosa, weobtained 82 isolates from ICU,57 &25 A .baumannii and P. aeruginosa respectively. Among these 57A. baumannii isolates 89.47%isolates were resistant to Ceftazidime and among these 33.33%isolates were ESBL producers.Of 25 P.aeruginosa isolatesobtained from ICU84 % were found to beresistant to ceftazidime by antibiotic sensitivity testing.Among these44% were ESBL producers.
Conclusion: Our results showed high prevalence ofNon fermenting gram negative bacilli in ICU patient’s samples, which were multidrug resistant and producers of Extended spectrum Beta lactamase enzymes.
Downloads
References
2. Gupta V. An update on newer beta-lactamases. Indian J Med Res. 2007 Nov;126(5):417-27.[pubmed]
3. AgarawalP,Gosh AN, Kumar S , Basu B, Kapila K. Prevalence of extended spectrum beta lactamases among E.coli and Klebsiella pneumoniae isolates in a tertiary care hospital. IJPM 2008;51(1):139-142.DOI:10.4103/0377-4929.40428.
4. J.G Colle and W. Marr. Specimen collection, culture containers and media,” in Mackie and McCartney Practical Medical Microbiology, G.J Collee, G.A. Fraser, B.P Marmon and A. Simmons, Eds., pp.14-27, Elseviar, Toronto, Canada,14 edition, 2008.
5. J.G Colle ,R.S Miles and B.Watt. Test for identification of bacteria,” in Mackie and McCartney Practical Medical Microbiology, G.J Collee, G.A. Fraser, B.P Marmon and A. Simmons, Eds., pp.131-150 ,Elseviar, Toronto, Canada,14 edition, 2008.
6. Clinical Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Vol. 32. Clinical Laboratory Standard Institute; Wayne, Pennsylvania, USA: 2012. pp. 70–71.
7. Koneman EW, Allen SD, Jand WM, Schreckenberg PC. Colour Atlas and Text Book of Diagnostic Microbiology. 6th ed. San Francisco: Lippincott;2006. p. 955‑63.
8. National treatment guidelines for Antimicrobial use in Infectious Diseases, version 1.0 National Centre for Disease Control; 2016;42.
9. Uma Karthika R, Srinivasa Rao R, Sahoo S, et al. Phenotypic and genotypic assays for detecting the prevalence of metallo-beta-lactamases in clinical isolates of Acinetobacter baumannii from a South Indian tertiary care hospital. J Med Microbiol. 2009 Apr;58(Pt 4):430-5. doi: 10.1099/jmm.0.002105-0.[pubmed]
10. Goel. N, Punia. P, Chaudhary U. Prevalence of ESBL, MBL and Amp C producing XDR Acinetobacter isolates from lower respiratory tract specimens. International Journal of Contemporary Medical Research 2017;4(10):2091-2095.
11. Manikal VM, Landman D, Saurina G, et al. Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin Infect Dis. 2000 Jul;31(1):101-6. Epub 2000 Jul 17. DOI:10.1086/313902.[pubmed]
12. Agrawal G, Lodhi R B , Kamalakar U P , Khadse R K , Jalgaonkar S V ,Study of Metallobeta lactamase production in clinical isolates of Pseudomonas aeruginosa,IJMM2008 ;26(4):349-51.DOI:10.4103/0255-0857.43573 PMID:18974488.
13. Sarkar B, Biswas D, Prasad R, et al. A clinicomicrobiological study on the importance of pseudomonas in nosocomially infected ICU patients, with special reference to metallo beta1-lactamase production. Indian J Pathol Microbiol. 2006 Jan;49(1):44-8.[pubmed]
14. Goel V, Hogade SA, Karadesai SG. Prevalence of extended-spectrum beta-lactamases, AmpC beta‑lactamase, and metallo‑beta‑lactamase producing Pseudomonas aeruginosa and Acinetobacter baumannii in an intensive care unit in a tertiary Care Hospital. J Sci Soc 2013;40(1):28-31. DOI:10.4103/0974-5009.109691.
15. Aggarwal R, Chaudhary U, Bala K. Detection of extended‑spectrum beta‑lactamase in Pseudomonas aeruginosa. Indian J Pathol Microbiol 2008;51(2):222‑4.DOI:10.4103/0377-4929.41693.
16. Joshi SG, Litake GM, Ghole VS, et al. Plasmid-borne extended-spectrum beta-lactamase in a clinical isolate of Acinetobacter baumannii. J Med Microbiol. 2003 Dec;52(Pt 12):1125-7. DOI:10.1099/0022-1317-52-12-1125.
17. Abd El-Fattah SM. Evaluation of antibiotic resistance among Gram-ve bacilli isolated from critically ill patients: Relation to risk factors and liberal use of antibiotics. M.Sc Thesis in Medical Microbiology and Immunology. Faculty of Medicine. Giza, Egypt: Cairo University; 2008.[pubmed]


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


