Differential mast cell density in spectrum of benign neoplasms of breast: potential for patient stratification and personalised treatment approach
Abstract
Background: Mast cells have a clear anti-tumorigenic role in invasive breast carcinoma. However, their role in the stepwise progression of benign fibroepithelial neoplasms of breast and mesenchymal tumours of the breast is less understood. Increased C-KIT (a receptor tyrosine kinase) expression in those tumours have been found to be due to presence of mast cells and a potential for patient stratification and individualized targeted therapy especially in phyllodes tumours. The present analysis was undertaken to assess the differential mast cell density within the spectrum of benign fibroepithelial neoplasms of the breast.
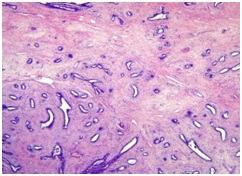
Methods: Tissue from fifty three cases of fibroadenoma and it variants including cellular fibroadenoma, fibroadenoma with increased stromal cellularityand benign phyllodes tumour were included in the study. Mast cells were clearly demonstrated in breast tissue using Toluidine Blue stain at pH 2.3. Mast cells were counted using an eyepiece grid and expressed as no. of cells / per sq. mm, i.e., mast cell density (MCD). Differential mast cell density in spectrum of benign breast neoplasms was analysed and statistical analysis was done.
Results: Mast cell density was statistically significantly higher in tissue from fibroadenoma compared to normal breast tissue. MCD was increased in cellular fibroadenoma and fibrodenoma with increased stromal cellularity compared to fibroadenoma. MCD was increased in benign phyllodes tumour compared to fibroadenoma with increased stromal cellularity, cellular fibroadenoma and fibroadenoma.
Conclusions: Our results indicate a relative and progressive increase in mast cells in fibroadenomas compared to normal breast tissue and comparatively increased with increased cellularity and increased stromal cellularity among the spectrum of fibroepithelial neoplasms. The study could be extended with larger sample size especially of phyllodes tumours and combined with immunohistochemical (IHC) methods (antibodies for CD 117) could be used for patient stratification and selection for personalized treatment including anti-C-KIT therapy.
Downloads
References
2. Mushtaq MU, Papadas A, Pagenkopf A, Flietner E, Morrow Z, Asimakopoulos F et al. Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers. J Immunother Cancer 2018. Jul 3;6(1):65. doi: 10.1186/s40425-018-0376-0.[pubmed]
3. Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol 2004; 25(5): 235–41.[pubmed]
4. Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol 2009; 39(1):11–25.[pubmed]
5. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357(9255):539–45.[pubmed]
6. Kirchhoff D, Bischoff SC, Maurer M, Zollner TM. Mast cells in health and disease: from basic science to clinical application 4-5 July 2008, Stuttgart, Germany. Expert OpinTher Targets 2008; 12(12):1591–4.[pubmed]
7. Crivellato E, Beltrami C alberto, Mallardi F, Ribatti D. Paul Ehrlich’s doctoral thesis: a milestone in the study of mast cells. Br J Haematol 2003; 123(1): 19–21.[pubmed]
8. Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Bajdik C, et al. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favourable prognosis. Mod Pathol Off J United States Can AcadPathol Inc 2004;17(6):690–5.[pubmed]
9. Sang J, Yi D, Tang X, Zhang Y, Huang T. The associations between mast cell infiltration, clinical features and molecular types of invasive breast cancer. Oncotarget. 2016 Dec 6;7(49):81661-81669. doi: 10.18632/oncotarget.13163.[pubmed]
10. Hussain MA, Tyagi SP, Tyagi N, Khan MH. Stromal cellular response in breast tumours and allied lesions. J Indian Med Assoc. 1992 May; 90(5):119-21.[pubmed]
11. Loke BN, Md Nasir ND, Thike AA, Lee JYH, Lee CS, Tan PH. Genetics and genomics of breast fibroadenomas. J Clin Pathol. 2018 May;71(5):381-387. doi: 10.1136/jclinpath-2017-204838. Epub 2017 Dec 16.[pubmed]
12. Combs JW, Purnell DM. Functional characteristics of mast cells associated with rat mammary tumors induced by 7,12-dimethylbenz(a) anthracene. J Natl Cancer Inst. 1973 Apr; 50(4):1003-11.[pubmed]
13. Yang X1, Kandil D, Cosar EF, Khan A. Fibroepithelial tumors of the breast: pathologic and immunohistochemical features and molecular mechanisms. Arch Pathol Lab Med. 2014 Jan;138(1):25-36. doi: 10.5858/arpa.2012-0443-RA.
14. Kashiwase Y, Morioka J, Inamura H, Yoshizawa Y, Usui R, Kurosawa M. Quantitative analysis of mast cells in benign and malignant breast lesions. Immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Int Arch Allergy Immunol. 2004 Jul;134(3):199-205. Epub 2004 Jun 1.[pubmed]
15. Djordjevic B, Hanna WM. Expression of c-kit in fibroepithelial lesions of the breast is a mast cell phenomenon. Mod Pathol. 2008 Oct;21(10):1238-45. doi: 10.1038/modpathol.2008.78. Epub 2008 May 23.[pubmed]
16. Vilela MH, de Almeida FM, de Paula GM, Ribeiro NB, Moreira MA et al. Utility of Ki-67, CD10, CD34, p53, CD117, and mast cell content in the differential diagnosis of cellular fibroadenomas and in the classification of phyllodes tumors of the breast. Int J SurgPathol. 2014 Sep; 22(6):485-91. doi: 10.1177/1066896914521290. Epub 2014 Feb 3.[pubmed]
17. Kondi-Pafiti A, Arkadopoulos N, Gennatas C, Michalaki V, Frangou-Plegmenou M, Chatzipantelis P. Expression of c-kit in common benign and malignant breast lesions. Tumori. 2010 Nov-Dec; 96(6):978-84.[pubmed]
18. CD117 and CD34 staining patterns in childhood benign mammary lesions. Kaçar A, Paker I, Akbiyik F, Arikök AT, Mambet E. Turk PatolojiDerg. 2012; 28(1):31-7. doi: 10.5146/tjpath.2012.01094.[pubmed]
19. Bose P, Dunn ST, Yang J, Allen R, El-Khoury C et al. c-Kit expression and mutations in phyllodes tumors of the breast. Anticancer Res. 2010 Nov; 30(11):4731-6. 44.[pubmed]
20. Sridharan G, Shankar AA. Toluidine blue: A review of its chemistry and clinical utility. J Oral MaxillofacPathol JOMFP 2012;16(2):251–5.[pubmed]
21. Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med1992;21(4):160–3.[pubmed]


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


