Comparative evaluation of Bethesda system with conventional reporting method in assessment of thyroid malignancy rate
Abstract
Introduction: Fine needle aspiration cytology (FNAC) of thyroid is non-invasive, cost-effective & extremely useful technique in identifying a substantial proportion of thyroid nodules as benign & appropriately triages patients with thyroid cancer to appropriate surgery. The Bethesda system for reporting thyroid cytology represents a major step towards improved clinical significance, reproducibility, standardization & greater predictive value of FNAC thyroid.
Objective: To categories thyroid lesions according to Bethesda system & to determine the rate of malignancy in each cytologic category by correlating the result with histopathologic diagnosis on the resected specimens.
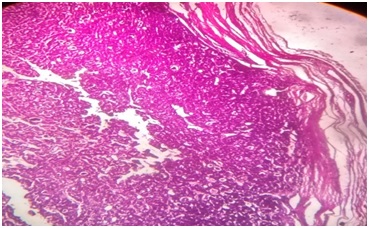
Material & Methods: The present study was carried out on 100 patients of thyroid swelling referred to the department of pathology, for FNAC. The reporting was done by using Bethesda system of thyroid cytopathology in comparison to the conventional reporting system. The malignancy rate based on final histopathologic evaluation was analyzed for each of the cytologic groups.
Results: Rates of malignancy in each category of conventional system reported on follow up HPE were 16.66% in non-diagnostic group, 2.17% in colloid garter, nil in colloid cyst, 25% in thyroiditis, 7.69% in follicular lesion/ neoplasm, 33.33% in indeterminate group, 75% in suspicious for malignancy & 100% in malignant category. Rates of malignancy in each category of Bethesda system on follow up HPE were 16.66% in Non Diagnostic, 1.58% in benign category, 28.57% in atypical of undermined significance, 18.18% in suspicious for follicular neoplasm, 80% in suspicious for malignancy & 100% in malignant category.
Conclusion: By adapting the Bethesda cyto-pathology reporting system malignancy risk in the categories of AFLUS, SM & Malignant category help to determine a better patient outcome due to proper clinical management.
Downloads
References
2. Moslavac S, Matesa N, Kusić Z. Thyroid fine needle aspiration cytology in children and adolescents. Coll Antropol. 2010 Mar;34(1):197-200.[pubmed]
3. Shagufta Tahir Mufti, Rihab Monh. The Bethesda system for reporting thyroid cytopathology A five years retrospective review of one center experience. Int. J. health Sci(Qassim), 2012 June; 6(2) : 159-173.
4. Bukhari MH, Niazi S, Hanif G, et al. An updated audit of fine needle aspiration cytology procedure of solitary thyroid nodule. Diagn Cytopathol. 2008 Feb;36(2):104-12. doi: 10.1002/dc.20731.[pubmed]
5. Santosh Kumar Mondal, Simant Sinha, Bijan Basak, Dipanwita Nag Roy & Swapan Kumar Sinha. The Bethesda System for reporting thyroid fine needle aspirates. Journal of cytology, Vol.30,No.2,April-June 2013,94-99. Doi:10.4103/0970-9371.112650
6. Langer JE, Baloch ZW, McGrath C, et al. Thyroid nodule fine-needle aspiration. Semin Ultrasound CT MR. 2012 Apr;33(2):158-65. doi: 10.1053/j.sult.2011.12.002.[pubmed]
7. Edmund S. Cibas,MD, and Syed Z Ali, MD. The Bethesda system for reporting thyroid cytopathology AM.J.Clipathol 2009;132:658-665.[pubmed]
8. Baloch ZW, Cibas ES, Clark DP, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal. 2008 Apr 7;5:6. doi: 10.1186/1742-6413-5-6.[pubmed]
9. Yassa L, Cibas ES, Benson CB, Frates MC, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007 Dec 25;111(6):508-16.[pubmed]
10. Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007 Oct 25;111(5):306-15.[pubmed]
11. Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009 Jun 25;117(3):195-202. doi: 10.1002/cncy.20029.[pubmed]


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


