Evaluation of GeneXpert MTB/RIF assay in diagnosis of extrapulmonary tuberculosis and rifampicin resistance
Abstract
Background: The prevalence of tuberculosis is still on the rise particularly extrapulmonary cases. There is an urgent need for rapid diagnosis of these cases for prompt initiation of treatment.
Aim: To evaluate the role of GeneXpert MTB/RIF Assay in the diagnosis of clinically suspected extrapulmonary tuberculosis (EPTB) and to detect rifampicin resistance in these cases.
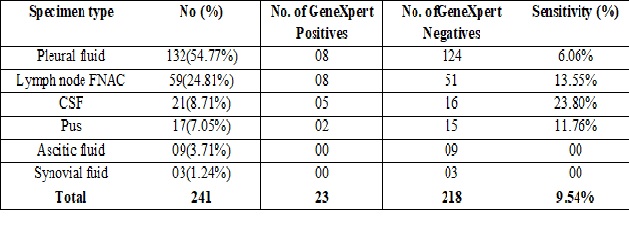
Materials and methods: A total of 241 samples from April 2016 to July 2018, from all clinically suspected EPTB patients with support of either laboratory or radiological evidence were included in study. 132 pleural fluid, 59 lymph node aspirate, 21 CSF, 17 pus, 9 ascitic fluid and 3 synovial fluid samples were screened for presence of acid fast bacilli (AFB) by conventional Zeihl-Neelsen (ZN) technique. The same samples were also used for testing by GeneXpert MTB/RIF Assay.
Results: Out of 241 EPTB samples tested, none were positive for AFB by ZN staining. Overall 9.54 % (23 out of 241) samples were positive for MTB by GeneXpert with a positivity rate of 23.80%, 13.55%, 11.76% & 6.06% respectively for CSF, lymph node aspirate, pus and pleural fluid. MTB was not detected in any of ascitic fluid & synovial fluid samples. Of the 23 MTB positive samples detected by GeneXpert, all were sensitive to rifampicin.
Conclusions: GeneXpert was found to be simple, effective and useful diagnostic method for detection of EPTB due to its rapidity & simultaneous detection of rifampicin resistance, thus reducing the time taken for initiation of treatment.
Downloads
References
2. Avashia S, Bansal D, Ahuja K, Agrawal V. Comparison of conventional methods with gene xpertmtb/rif assay for rapid detection of mycobacterium tuberculosis and rifampicin resistance in extra-pulmonary samples. Int J Med Res Rev 2016;4(2):181-185. doi: 10.17511/ijmrr.2016.i02.010.
3. Hall GS. Primary processing of specimens and isolation and cultivation of mycobacteria. Clin Lab Med. 1996 Sep;16(3):551-67.[pubmed]
4. Park C, Hixon D, Furguson C. Rapid recovery of mycobacteria from clinical specimens using automated radiometric technique. Am J Clin Path1984; 81:341-5.
5. Piatek AS, Tyagi S, Pol AC, et al. Molecular beacon sequence analysis for detecting drug resistance in Mycobacterium tuberculosis. Nat Biotechnol. 1998 Apr;16(4):359-63. DOI:10.1038/nbt0498-359.[pubmed]
6. World Health Organization (2011) Automated realtime nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Policy statement.
7. Arora VK, Chopra KK. Extra pulmonary tuberculosis. Indian J Tuberc. 2007 Oct;54(4):165-7.[pubmed]
8. Gour SM, Munje RP, Babu NP, Sushant M, Jites A. Genotypic diagnosis of extra pulmonary tuberculosis – CBNAAT a novel tool.MedPulse International Journal of Medicine. November 2017; 4(2):79-82.doi:10.26611/1021425
9. Chander V, Raina SK, Bhardwaj AK, Kashyap S, Gupta AK, Sood A. Clinico-Epidemiological Profile of extra Pulmonary Tuberculosis: A Report from a High Prevalence State of Northern India. Public Health Research 2012, 2(6): 185-189.doi:10.5923/j.phr.20120206.02
10. Prakasha SR, Suresh G, D'sa IP, et al. Mapping the pattern and trends of extrapulmonary tuberculosis. J Glob Infect Dis. 2013 Apr;5(2):54-9. doi: 10.4103/0974-777X.112277.[pubmed]
11. Pravin KN and Chourasia E. Use of GeneXpert Assay for Diagnosis of Tuberculosis From Body Fluid Specimens, a 2 Years Study . J Microbiol Biotechnol, 2016, 1(1): 000105.
12. Causse M, Ruiz P, Gutiérrez-Aroca JB, Casal M. Comparison of two molecular methods for rapid diagnosis of extrapulmonary tuberculosis. J Clin Microbiol. 2011 Aug;49(8):3065-7. doi: 10.1128/JCM.00491-11. Epub 2011 Jun 8.[pubmed]
13. Hillemann D, Rüsch-Gerdes S, Boehme C, Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. DOI:10.1128/JCM.02268-10.[pubmed]
14. Rufai SB, Singh A, Kumar P, et al. Performance of Xpert MTB/RIF Assay in Diagnosis of Pleural Tuberculosis by Use of Pleural Fluid Samples. J Clin Microbiol. 2015 Nov;53(11):3636-8. doi: 10.1128/JCM.02182-15. Epub 2015 Aug 26.[pubmed]
15. Porcel JM, Palma R, Valdés L, et al. Xpert® MTB/RIF in pleural fluid for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2013 Sep;17(9):1217-9. doi: 10.5588/ijtld.13.0178. Epub 2013 Jul 3.[pubmed]
16. Sharma SK, Ryan H, Khaparde S, et al. Index-TB guidelines: Guidelines on extrapulmonary tuberculosis for India. Indian J Med Res. 2017 Apr;145(4):448-463. doi: 10.4103/ijmr.IJMR_1950_16.[pubmed]
17. Alvarez-Uria G, Azcona JM, Midde M, et al. Rapid Diagnosis of Pulmonary and Extrapulmonary Tuberculosis in HIV-Infected Patients. Comparison of LED Fluorescent Microscopy and the GeneXpert MTB/RIF Assay in a District Hospital in India. Tuberc Res Treat. 2012;2012:932862. doi: 10.1155/2012/932862. Epub 2012 Aug 26.
18. Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014 Aug;44(2):435-46. doi: 10.1183/09031936.00007814. Epub 2014 Apr 2.[pubmed]
19. Tortoli E, Russo C, Piersimoni C, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J. 2012 Aug;40(2):442-7. doi: 10.1183/09031936.00176311. Epub 2012 Jan 12.[pubmed]
20. Nhu NT, Heemskerk D, Thu do DA, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014 Jan;52(1):226-33. doi: 10.1128/JCM.01834-13. Epub 2013 Nov 6.[pubmed]
21. Patel VB, Theron G, Lenders L, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med. 2013 Oct;10(10):e1001536. doi: 10.1371/journal.pmed.1001536. Epub 2013 Oct 22.[pubmed]
22. Kumar A, Das S, Paul DK. A Study on the Role of Cartridge Based Nucleic Acid Amplification Test (CBNAAT) for Diagnosing Pediatric Tuberculosis in a Tertiary Care Hospital in Eastern India. Acad J PedNeonatol. 2018; 6(3): 555745. doi: 10.19080/AJPN.2018.06.555745.
23. Munir MK, Shabbir I, Iqbal R, Khan SU. Comparison of AFB Smear Microscopy and Culture from Specimens Received for the Diagnosis of Extra Pulmonary Tuberculosis. P J M H S 2009; 1(1):59-61.
24. Bagdia M, Bijwe S, Hirani N, Joshi A, Chowdhary A, Agrawal M, Bagdia A. Lab Diagnosis of Extra Pulmonary Tuberculosis: Comparison of Histopathology, Cytology, ZeihlNeelsen stain and Light Emission Diode Microscopy with Culture and Nucleic Acid Amplification Tests.Int J Cur Res Rev. 2018 Apr; 10(8).15-19.
25. Banada PP, Sivasubramani SK, Blakemore R, et al. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol. 2010 Oct;48(10):3551-7. doi: 10.1128/JCM.01053-10. Epub 2010 Aug 18.[pubmed]
26. Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014 Aug;44(2):435-46. doi: 10.1183/09031936.00007814. Epub 2014 Apr 2.[pubmed]
27. Tortoli E, Russo C, Piersimoni C, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J. 2012 Aug;40(2):442-7. doi: 10.1183/09031936.00176311. Epub 2012 Jan 12.[pubmed]
28. Nhu NT, Heemskerk D, Thu do DA, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014 Jan;52(1):226-33. doi: 10.1128/JCM.01834-13. Epub 2013 Nov 6.
29. Patel VB, Theron G, Lenders L, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med. 2013 Oct;10(10):e1001536. doi: 10.1371/journal.pmed.1001536. Epub 2013 Oct 22.[pubmed]
30. Kumar A, Das S, Paul DK. A Study on the Role of Cartridge Based Nucleic Acid Amplification Test (CBNAAT) for Diagnosing Pediatric Tuberculosis in a Tertiary Care Hospital in Eastern India. Acad J PedNeonatol. 2018; 6(3): 555745. doi: 10.19080/AJPN.2018.06.555745.
31. Munir MK, Shabbir I, Iqbal R, Khan SU. Comparison of AFB Smear Microscopy and Culture from Specimens Received for the Diagnosis of Extra Pulmonary Tuberculosis. P J M H S 2009; 1(1):59-61.
32. Bagdia M, Bijwe S, Hirani N, Joshi A, Chowdhary A, Agrawal M, Bagdia A. Lab Diagnosis of Extra Pulmonary Tuberculosis: Comparison of Histopathology, Cytology, ZeihlNeelsen stain and Light Emission Diode Microscopy with Culture and Nucleic Acid Amplification Tests.Int J Cur Res Rev. 2018 Apr; 10(8).15-19.
33. Banada PP, Sivasubramani SK, Blakemore R, et al. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol. 2010 Oct;48(10):3551-7. doi: 10.1128/JCM.01053-10. Epub 2010 Aug 18.[pubmed]


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


