Histopathological study of lower gastrointestinal tract lesions
Patel V.1*, Goyal A.2
DOI: https://doi.org/10.17511/jopm.2021.i04.07
1* Vibhaben Kantilal Patel, MD Pathology, Smt. NHL Municipal Medical College, V S. General Hospital, Ahmedabad, Gujarat, India.
2 Anjali Deepak Goyal, Associate Professor, Smt. NHL Municipal Medical College, V S General Hospital, Ahmedabad, Gujarat, India.
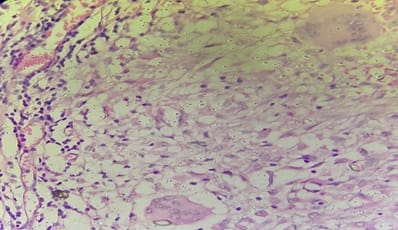
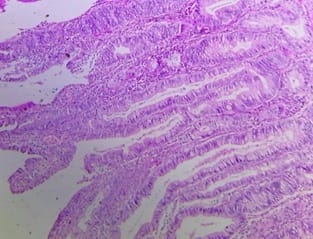
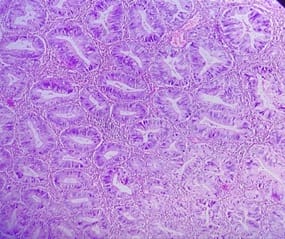
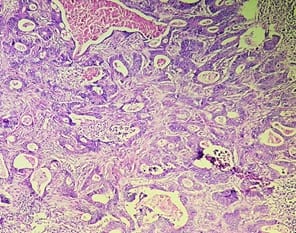
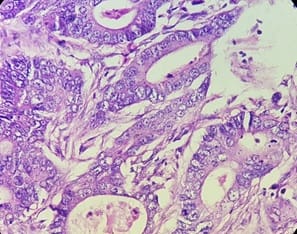
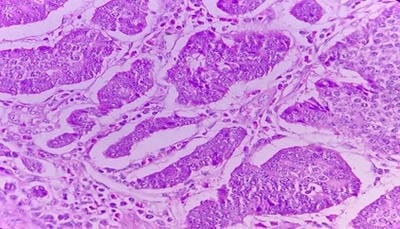
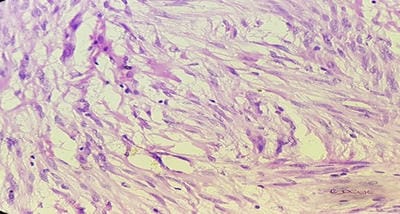
Background: The benign lesions of the lower gastrointestinal tract are responsible for a large number of morbidities. The microscopic examination of and determination of histological types of malignant lesions help to decide treatment options and to predict prognosis. The histopathological study is the Gold standard for the diagnosis of intestinal lesions. Aims and Objectives: To study the prevalence of various lower gastrointestinal tract lesions site-wise, age-wise and gender-wise and to compare the obtained results with other studies. Materials and methodology: A retrospective study of 600 various lower gastrointestinal tract lesions sent for histopathological examination at Pathology department of tertiary care centre, VS General Hospital, Ahmedabad is carried out. Results: Among all the 600 cases, non-neoplastic lesions 572 (95.34%) are far more common than neoplastic lesions 28 (4.66%). Conclusion: Non-neoplastic lesions are common in the small intestine, while the large intestine harbors most malignant lesions.
Keywords: Lower gastrointestinal tract, Malignant, Non-malignant
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , MD Pathology, Smt. NHL Municipal Medical College, V S. General Hospital, Ahmedabad, Gujarat, India. Email: |
Vibhaben Kantilal Patel, Anjali Deepak Goyal, Histopathological study of lower gastrointestinal tract lesions. Trop J Pathol Microbiol. 2021;7(4):194-200. Available From https://pathology.medresearch.in/index.php/jopm/article/view/557 |


 ©
©