Cutaneous cystic lesions: its clinicopathological correlation with emphasis on unusual findings.
Inbasekaran P.1*, Ramachandran T.2, Sivadharshini S.J.3, Murugan R.4
DOI: https://doi.org/10.17511/jopm.2021.i03.07
1* Poovizhi Inbasekaran, Assistant Professor, Department of Pathology, Vinayaka Missions Kirupananda Variyar Medical College and Hospital, Salem, Tamil Nadu, India.
2 Thamilselvi Ramachandran, Professor and Head, Department of Pathology, Vinayaka Missions Kirupananda Variyar Medical College and Hospital, Salem, Tamil Nadu, India.
3 Sivadharshini S.J, Senior Resident, Department of Pathology, Vinayaka Missions Kirupananda Variyar Medical College and Hospital, Salem, Tamil Nadu, India.
4 Roopmala Murugan, Associate Professor, Department of Pathology, Vinayaka Missions Kirupananda Variyar Medical College and Hospital, Salem, Tamil Nadu, India.
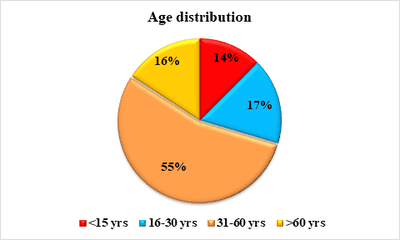
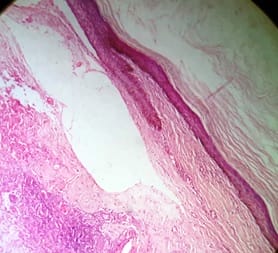
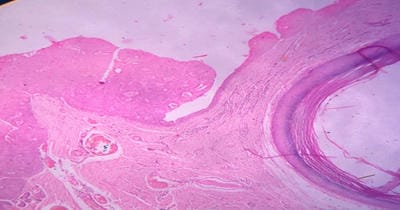
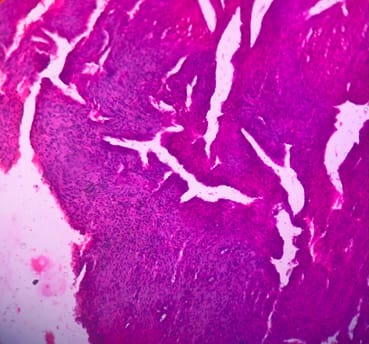
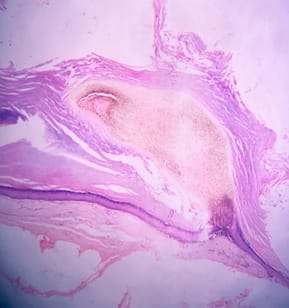
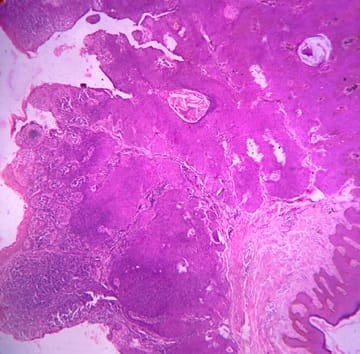
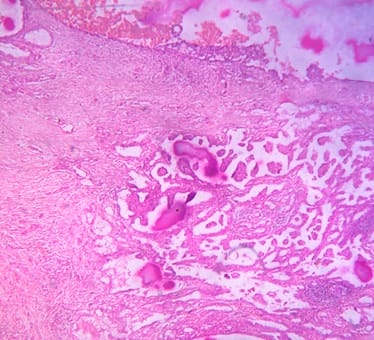
Background: Cutaneous cysts are the most common lesions encountered in surgical practice with cosmetic and psychological concern to the patient. Aim: To establish the clinicopathological correlation of these lesions and also the importance of histological evaluation for the prevention of misdiagnosing a benign-appearing malignant lesion. Methods: A retrospective descriptive study with data collected from archives of histopathology from January 2018 to December 2020 of clinically diagnosed cutaneous cyst along with age, gender, location and histopathological evaluation were analysed. Results: 88 cases that were clinically diagnosed as cutaneous cysts had a higher female proportion 52.3%. The most common clinical diagnosis is epidermal/ sebaceous cyst. 35.2% of cases were clinically diagnosed accurately but 64.8% of cases did not correlate with clinical diagnosis. 4 malignant lesions were misdiagnosed as benign cutaneous cyst clinically. Conclusion: Most often clinically diagnosed cutaneous cysts are not sent for histopathological evaluation which is a very important tool to confirm the diagnosis.
Keywords: Cutaneous cyst, Misdiagnosed, Malignant lesion
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Pathology, Vinayaka Missions Kirupananda Variyar Medical College and Hospital, Salem, Tamil Nadu, India. Email: |
Inbasekaran P, Ramachandran T, Sivadharshini SJ, Murugan R. Cutaneous cystic lesions: its clinicopathological correlation with emphasis on unusual findings.. Trop J Pathol Microbiol. 2021;7(3):135-143. Available From https://pathology.medresearch.in/index.php/jopm/article/view/539 |


 ©
©