Comparison of various principles of coagulation tests in handling hemolysed blood samples
Vani K.1*, Ahmad R.2
DOI: https://doi.org/10.17511/jopm.2021.i04.06
1* Vani K, Associate professor, Department of Pathology, JSS Medical College, JSS Academy of higher education and research, Mysuru, Karnataka, India.
2 Rubaiya Ahmad, Tutor, Shrikrishna Medical College & Hospital, Muzaffarpur, Bihar, India.
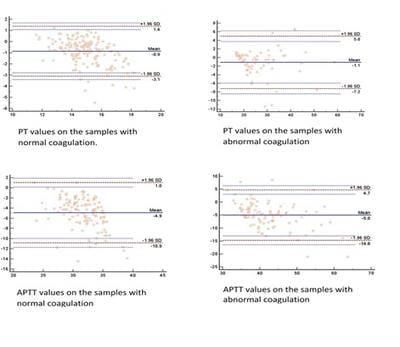
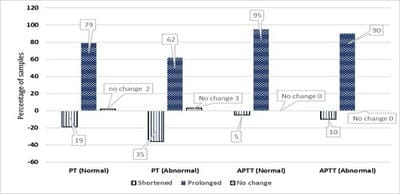
Background: Rejection of hemolysed samples for coagulation test is the standard practice. However, when clinicians deal with extremely sick patients where repeat sampling is difficult to obtain, rejection of the sample is a lost opportunity for the lab physician to assist inpatient care. Proceeding with the test and providing a clinically helpful interpretation of the results will ensure the active participation of the laboratory physician. Different principles of coagulation testing handle the hemolysed samples differently. It is essential to know the best principle to proceed with the hemolysed sample if need be. This study set out to estimate the predictive values of post-hemolytic sample coagulation test results with various coagulation test principles. Methods: This is a prospective experimental study where the non-hemolysed samples were processed for coagulation tests. Part of the sample was deliberately hemolysed, and the coagulation tests were repeated. Results: Two hundred and forty-eight samples were studied. A median of 11% hemolysis was achieved experimentally. The mean difference in prothrombin time between pre and post hemolytic samples with normal PT was 0.9 and with abnormal PT, it was 1.1 seconds. The same for APTT was 4.9 and 1.1 seconds, respectively. The majority of the samples showed prolonged coagulation post hemolysis. Positive (PPV) and negative (NPV) predictive values for prothrombin time are 97.3 and 73.4%, respectively. Similarly, PPV and NPV for APTT are 97.4 and 47.1%, respectively. Conclusions: Samples with normal values after hemolysis are more likely to be normal.
Keywords: Hemolysis, Prothrombin Time, Coagulation Tests, Partial Thromboplastin Time
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate professor, Department of Pathology, JSS Medical College, JSS Academy of higher education and research, Mysuru, Karnataka, India. Email: |
Vani K, Ahmad R. Comparison of various principles of coagulation tests in handling hemolysed blood samples. Trop J Pathol Microbiol. 2021;7(4):188-193. Available From https://pathology.medresearch.in/index.php/jopm/article/view/534 |


 ©
©