Epidemiology of Mycotic Infections: Experience From A Tertiary Care Center Of Uttarakhand, India
Mohanty A.1, Rohilla R.2*, Meena S.3, Bora M.4, Singh A.5, Kaistha N.6, Gupta P.7
DOI: https://doi.org/10.17511/jopm.2021.i03.01
1 Aroop Mohanty, M.D, Assistant Professor, 2* Ranjana Rohilla, Assistant professor, Department of Microbiology, Shri Guru Ram Rai Institute of Medical & Health Sciences, Dehradun, Uttarakhand, India. 3 Suneeta Meena, M.D, Assistant Professor, Department of Laboratory Medicine, All India Institute of Medical Sciences, Delhi, India. 4 Mamta Bora, Student M.Sc, Department of Medical Microbiology, Uttaranchal PG College of Biomedical Sciences and Hospital, Rishikesh, Uttarakhand, India. 5 Anshu Singh, Ph.D. scholar, 6 Neelam Kaistha, M.D. Professor, 7 Pratima Gupta, M.D. Professor, 1,5,6,7authors are affiliated with the Department of Microbiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India.
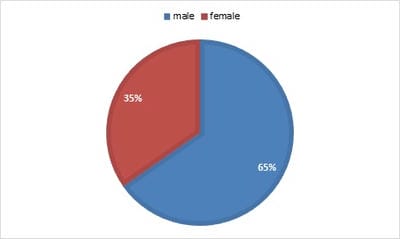
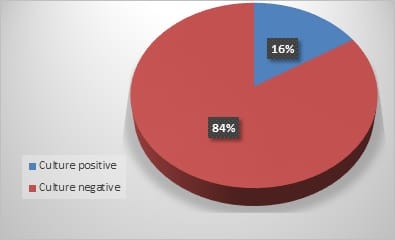
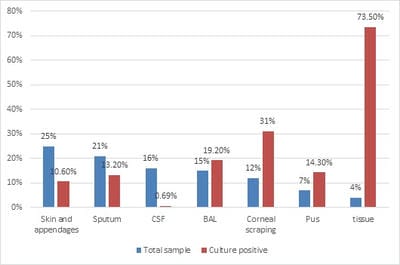
Introduction: The overall changing epidemiology of fungal infections in the current scenario is because of an increase in immunocompromised population including cancer patients, Human immunodeficiency virus (HIV)-infected patients, transplant receipts, and prolonged hospitalization with overuse of antimicrobial agents. These infections are challenging to diagnose and subsequently manage as their clinical symptomatology often mimics other common diseases like tuberculosis. Rapid diagnosis is limited and culture is often delayed due to slow growth rates of the causative agents. Objective: This is a retrospective study to know the spectrum and burden of mycotic infections in a tertiary care hospital. Methods: All samples collected from clinically suspected cases of fungal infections were sent to the Microbiology department over one year. The common specimens received were respiratory samples, scrapings from cornea, skin, and nail. All samples were first observed under direct microscopy using Potassium hydroxide (KOH) examination for the presence of fungal elements and Gram stain for yeasts. India Ink examination was performed for sterile fluids. Fungal culture was done on Sabouraud's dextrose agar. Result: A total of 900 samples from various departments were included, KOH examination was positive for 380 samples (42%) and fungal growth was obtained in 144 samples (16%). Rare fungi like Trichosporon dohaense (blood culture), Cladophialophora bantiana (brain abscess), Scedosporium apiospermum and Candida auris (blood culture) were also isolated. Conclusion: Similar studies are needed to estimate the actual burden of the fungal infections in tertiary care health facilities, to help decrease the morbidity and mortality associated with underdiagnosed mycotic infections.
Keywords: Mycotic infections, Fungal spectrum, Fungal culture, Rare fungi
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant professor, Department of Microbiology, Shri Guru Ram Rai Institute of Medical & Health Sciences, Dehradun, Uttarakhand, India. Email: |
Mohanty A, Rohilla R, Meena S, Bora M, Singh A, Kaistha N, Gupta P. Epidemiology of Mycotic Infections: Experience From A Tertiary Care Center Of Uttarakhand, India. Trop J Pathol Microbiol. 2021;7(3):93-98. Available From https://pathology.medresearch.in/index.php/jopm/article/view/525 |


 ©
©