The Spectrum of Malignant Breast lesions by Fine Needle Aspiration Cytology in a Teaching Hospital
Alexander M.1, A. Pattanashetti M.2*
DOI: https://doi.org/10.17511/jopm.2021.i04.05
1 Manika Alexander, MBBS, MD Pathology, Associate Professor, Department of Pathology, Gadag Institute of Medical Sciences, Gadag, Karnataka, India.
2* Mallikarjun A. Pattanashetti, MBBS, MD Pathology, Assistant Professor, Department of Pathology, Gadag Institute of Medical Sciences, Gadag, Karnataka, India.
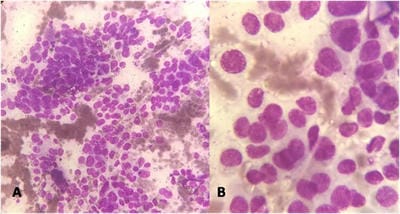
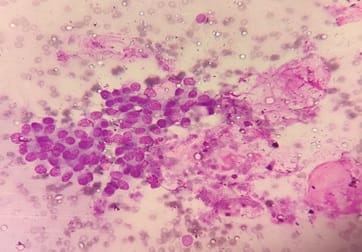
Background: Fine needle aspiration cytology (FNAC) is one of the preliminary tests done to detect malignant breast lesions, which help in early detection and management. Studying the cytology features of various malignant breast diseases was the aim of this study. Methods: This study is a cross-sectional retrospective study conducted in the Department of Pathology from 2015 to 2020. Clinical details and cytology features were collected from the Department records. Results: A total of 75 cases were collected during the study period. All the cases were females. The spectrum of lesions was composed of Ductal carcinoma followed by one point each of Mucinous carcinoma, Malignant Phyllodes tumour and Lobular Carcinoma. Conclusions: FNAC helps in rapid diagnosis and early management of malignant breast lesions.
Keywords: Breast cytology, Malignant breast lesions, FNAC
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , MBBS, MD Pathology, Assistant Professor, Department of Pathology, Gadag Institute of Medical Sciences, Gadag, Karnataka, India. Email: |
Alexander M, Pattanashetti MA. The Spectrum of Malignant Breast lesions by Fine Needle Aspiration Cytology in a Teaching Hospital. Trop J Pathol Microbiol. 2021;7(4):181-187. Available From https://pathology.medresearch.in/index.php/jopm/article/view/519 |


 ©
©