Relationship of Neutrophil Lymphocyte Ratio (NLR) with Parathyroid Hormone (PTH) in Maintenance Hemodialysis Patients
Hazra A.1*, Mandal S.2, Chakraborty J.3
DOI: https://doi.org/10.17511/jopm.2021.i01.03
1* Asmita Hazra, Assistant Professor, Department of Biochemistry, Government Medical College, Pali, Rajasthan, India.
2 Saptarshi Mandal, Associate Professor, Department of Transfusion Medicine & Blood Bank, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India.
3 Jayati Chakraborty, Professor & Head, Department of Pathology, ESI-PGIMSR, Manicktala, Kolkata, India.
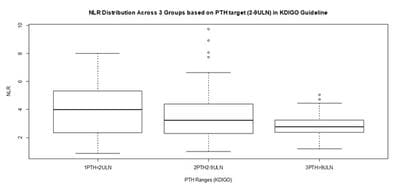
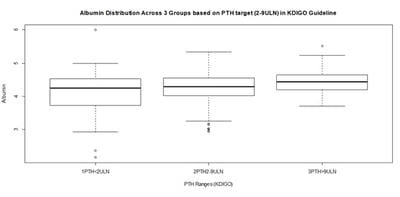
Background: Neutrophil-Lymphocyte-Ratio (NLR), a simple derived parameter of routine Complete Blood Counts (CBC), has been shown to be better than WBC count as a marker for inflammation in many contexts. Inflammation is associated with adverse outcomes in hemodialysis patients. Methods: A cross sectional study was designed on 100 maintenance hemodialysis Patients in a tertiary care hospital in Eastern India after IRB permission and informed consent. 88 complete results became available. Complete blood count (CBC), routine PTH and biochemistry analysis were performed. KDIGO 2009 cut offs were used to classify patients by PTH levels (PTH<2ULN, PTH 2-9ULN & PTH>9 ULN). NLR was calculated and NLR of 3.5 was used as high inflammation cut off. Results: In Analysis of 88 samples, significant Pearson Correlation with NLR was shown by Albumin, Hemoglobin, PTH, Gender, Age, and Sugar, but involved collinearities. Multiple Linear Regression with Robust estimation of Standard Errors retained only PTH as a significant predictor (Beta= -0.273, P=0.033) of NLR and Albumin as a borderline significant predictor (Beta= -0.501, P= 0.061). The population was partitioned into 3 sets based on PTH as per KDIGO guideline. Conclusion: Our study suggests that PTH is a significant predictor of inflammation as measured by NLR, independent of the other parameters, and it has an overall weak negative association with inflammation especially in the mid-range (PTH 2-9 ULN) subgroup, which largely corroborates with available but scant literature.
Keywords: Hemodialysis (HD), Neutrophil Lymphocyte Ratio (NLR), Parathyroid Hormone (PTH), Inflammation, Multiple Regression
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Biochemistry, Government Medical College, Pali, Rajasthan, India. Email: |
Hazra A, Mandal S, Chakraborty J. Relationship of Neutrophil Lymphocyte Ratio (NLR) with Parathyroid Hormone (PTH) in Maintenance Hemodialysis Patients. Trop J Pathol Microbiol. 2021;7(1):17-25. Available From https://pathology.medresearch.in/index.php/jopm/article/view/510 |


 ©
©