Unique Features of Hashimoto’s Thyroiditis in Coastal South India – a study from a tertiary care hospital
Asokan V.1, Rajendran K.2, Sudalaimuthu M.3*
DOI: https://doi.org/10.17511/jopm.2020.i08.02
1 Vinodhini Asokan, Post-Graduate, Department of Pathology, SRM Medical College Hospital and Research Centre, Faculty of Medicine, SRM Institute of Science and Technology, Chennai, Tamil Nadu, India.
2 Koshalya Rajendran, Senior Resident, Department of Pathology, SRM Medical College Hospital and Research Centre, Faculty of Medicine, SRM Institute of Science and Technology, Chennai, Tamil Nadu, India.
3* Muthu Sudalaimuthu, Associate Professor, Department of Pathology, SRM Medical College Hospital and Research Centre, Faculty of Medicine, SRM Institute of Science and Technology, Chennai, Tamil Nadu, India.
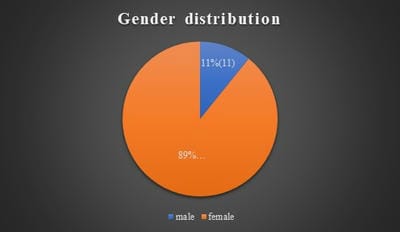
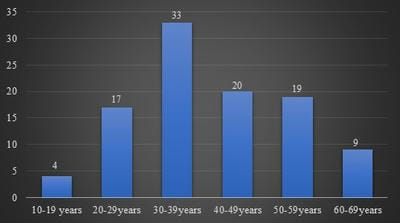
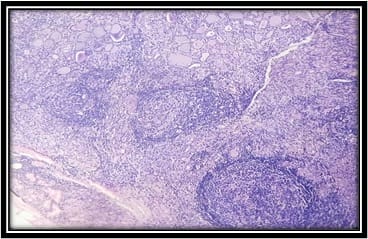
Background: Hashimoto’s thyroiditis is an autoimmune disorder of thyroid gland. It is one of the common causes of hypothyroidism and is common in females. Generally, Hashimoto’s thyroiditis clinically presents as diffuse enlargement of the thyroid and nodular lesions are uncommon. But few recent studies from South India have shown that Hashimoto’s frequently presents as nodular enlargement of the thyroid. Such lesions can be easily confused with nodular goitre. Objectives and Aim: Aim of the study is to study the clinicopathological features of Hashimoto’s thyroiditis and to estimate the frequency of nodular lesions in Hashimoto’s thyroiditis in a tertiary care health centre in coastal South India. Materials and Methods: The present study was done retrospectively on patients diagnosed as Hashimoto’s thyroiditis by fine-needle aspiration cytology during the period June 2017 to June 2020. Their clinical details, clinical examination findings including diffuse/nodular nature of the swelling, thyroid hormone status and ultrasound findings were studied. Results: In the present study, 102 cases of Hashimoto’s thyroiditis were included, which includes 91 females and 11 males. Patients age ranged from 15 to 63 years with a peak in the fourth decade. Fifty-five cases (53.9%) were hypothyroid and 43 (42.2%) were euthyroid. Fifty cases (49%) presented as nodular lesion out of which 47 cases had multiple nodules. Conclusion: Nodular enlargement of the thyroid is a common finding in Hashimoto’s thyroiditis patients. Such cases should not be mistaken for nodular goitre as there is a risk of malignancy in Hashimoto’s thyroiditis.
Keywords: Hashimoto’s thyroiditis, Hypothyroidism, Cytology, Histopathology
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pathology, SRM Medical College Hospital and Research Centre, Faculty of Medicine, SRM Institute of Science and Technology, Chennai, Tamil Nadu, India. Email: |
Asokan V, Rajendran K, Sudalaimuthu M. Unique Features of Hashimoto’s Thyroiditis in Coastal South India – a study from a tertiary care hospital. Trop J Pathol Microbiol. 2020;6(8):466-471. Available From https://pathology.medresearch.in/index.php/jopm/article/view/493 |


 ©
©