Analysis of antibiotic usage for surgical prophylaxis in a tertiary care hospital in Bangalore, Karnataka, India
Shashikala N.1, Manasa S.2*, Vikram HCV.3
DOI: https://doi.org/10.17511/jopm.2020.i04.08
1 Shashikala N., Infection Control Department, Sagar Hospital Banashankari, Bangalore, Karnataka, India.
2* Manasa S., Infection Control Department, Sagar Hospital Banashankari, Bangalore, Karnataka, India.
3 Venkatesh Vikram H.C., Medical Director, Sagar Hospital Banashankari, Bangalore, Karnataka, India.
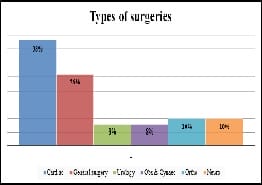
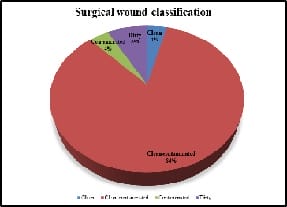
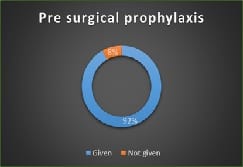
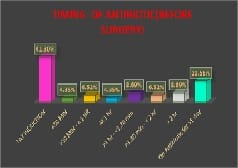
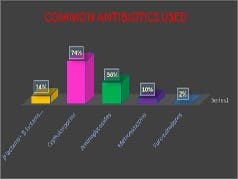
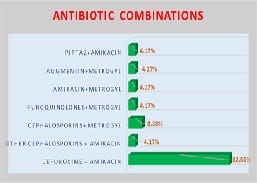
Introduction: Appropriately administered antibiotic prophylaxis reduces the incidence of surgical wound infection. The timing of antibiotic administration is very critical. The first dose should always be given before the procedure, preferably within 60 minutes before incision. Re-administration at one to two half-lives of the antibiotic is recommended for the duration of the procedure. In general, postoperative administration is not recommended except for cardiothoracic surgeries. Materials and Methods: This is a retrospective study done over a period of 6 months from January 2019 to June 2019 in a tertiary care hospital in Bangalore. A continuous 100 patients who underwent an elective surgery were audited regarding the antibiotic indication, choice, dosage, dosing interval, and timing of first antibiotic administration prior to skin incision and duration of prophylaxis were compared with the CDC guideline recommendations and hospital antibiotic policy. Results: A total of 100 surgeries were audited. Out of this, 4% were clean 84% were clean contaminated 4% were contaminated and 8% were dirty. The most commonly used antibiotics were cephalosporins 74% aminoglycosides 36%, β lactams 14%, and fluoroquinolones 2%. The three parameters tested for adherence showed individual compliance of 92% for appropriate selection of antibiotics, 85% for the appropriate administration, and 56% for the appropriate duration of antibiotics, respectively. Conclusion: The results highlight the challenges of disseminating evidence-based protocols systematically into routine clinical practice. Various measures are needed to improve appropriateness of prescriptions and adherence include the development of evidence-based guidelines in collaboration with surgeons, increased outcome-based research to document benefits of appropriate antibiotic use, continuing education to disseminate information to practitioners etc.
Keywords: Antibiotic prophylaxis, Guideline adherence, Surgery
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Infection Control Department, Sagar Hospital Banashankari, Bangalore, Karnataka, India. Email: |
Shashikala N, Manasa S, Venkatesh VHC. Analysis of antibiotic usage for surgical prophylaxis in a tertiary care hospital in Bangalore, Karnataka, India. Trop J Pathol Microbiol. 2020;6(4):313-323. Available From https://pathology.medresearch.in/index.php/jopm/article/view/453 |


 ©
©