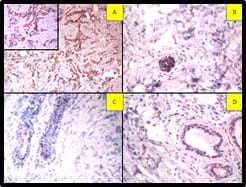
Fig-4: Tumour cells are positive for A. GCDFP (IHC, 400x), B. EMA (IHC, 400x), C. CK7(IHC, 400x) and Negative for Calretinin (IHC, 400x). Calretinin is positive in reactive mesothelial cells. (IHC has done on cell block preparation).
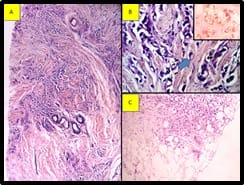
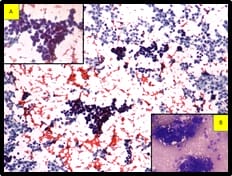
The pleural tap was done, revealed turbid hemorrhagic pleural fluid. Smears show features of metastatic adenocarcinoma (Figure 3). Immunocytochemistry was done on the cell block. Cells are positive for Cytokeratin 7 (CK 7), Gross Cystic Disease Fluid Protein-15(GCDFP), Epithelial membrane antigen (EMA), Carcinoembryonic antigen(CEA) and negative for Cytokeratin (CK20).
Calretinin is positive in reactive mesothelial cells (Figure 4). Correlating the morphology with the immunocytochemistry findings and history of carcinoma breast, the possibility of metastasis from the breast is to be considered.
Discussion
Breast carcinoma occurs in both genders. Although certain characteristics are similar for both genders, there are many differences in incidence, distribution of age, histological type, prognosis, and survival. Prognosis of male breast cancer is more unfavorable comparisons to the female patient because of presentation at an older age and advanced tumor stage at the time of diagnosis [1]. Our patients also presented at the age of 65 years.
The etiology of male breast cancer is unclear, however, there are many associations of breast cancer with testicular abnormalities such as undescended testes, orchitis, congenital inguinal hernia, and history of breast trauma [3].
Klinefelter’s syndrome is present in 3-7% of men with breast cancer, and it increases the breast cancer risk 50-fold greater than the general male patient [4]. Chest wall radiation for Hodgkin’s disease increases the subsequent cancer risk [3].
Pituitary prolactinoma also associated with male breast cancer, due to the effect of increased prolactin level which stimulates the breast tissue from premalignant to malignant. BRCA2 gene mutation carrier was more frequent, 4- 16% identified in male breast cancer patients [5].
Our case not associated with any of the above diseases. Although gynecomastia was very common in healthy men, it also has been reported in association with breast cancer [4,6,7]. Common
presenting symptoms of breast cancer patients are a painless subareolar lump, nipple retraction, and bleeding [8,9]. Our case also presents with swelling on both sides of the breast. As in females with breast cancer, male breast cancer also had a slight preponderance of the left side compared to the right side [10].
Data from a cancer registry in the Surveillance, Epidemiology, and End Results (SEER) in more than 2000 male patients show that ductal or unclassified carcinomas are common cancer identified in 93.7% of male breast cancer, remaining 2.6% are papillary,1.8% are mucinous and only 1.5% are lobular [1].
This distribution is in contrast to female breast cancer, in which around 12% of cancers are lobular carcinoma. The highest percentage of lobular carcinoma (4.2%) was noted with 612 male breast cancer from the Veterans Affairs Central Cancer Registry [11].
Hormonal receptors expression was higher in male breast cancer patients compared to female breast cancer and it also increases with age. Approximately, 90% of male breast cancer expressed the estrogen receptor and 81% expressed the progesterone receptor [1]. But, in contrast, the Her2 Neu receptor was less likely to be overexpressed in male breast cancer patients [12,13]. But our case is triple-negative.
Data from the Swedish Family-Cancer Database indicate male breast survivors had 93- fold greater risk of developing contralateral breast cancer compared to males without a history of breast cancer [14].
There was also the risk of developing other cancers, including melanoma and prostate cancer, particularly in mutation carriers [15]. Our patients had grade II prostatomegaly but PSA levelly is normal. Prostate biopsy not done for this patient.
Patients put on chemotherapy. But developed the sepsis during treatment and intubated due to respiratory distress and then subsequently died.
Conclusion
Bilateral male infiltrating lobular breast cancers was a very rare disease. So it is important to identify this type of breast cancer at an early stage and start the therapy as soon as possible for a better outcome.


 ©
©