Bacteriological profile and its antibiotic susceptibility in patients with urinary tract infection in tertiary care hospital
Mukherji T.1, Mukherji MB.2*
DOI: https://doi.org/10.17511/jopm.2020.i03.01
1 Tamasi Mukherji, Assistant Professor, Department of Microbiology, KPC Medical College and Hospital, Kolkata, West Bengal, India.
2* Mayur Bahan Mukherji, Associate Professor, Department of Medicine, KPC Medical College and Hospital, Kolkata, West Bengal, India.
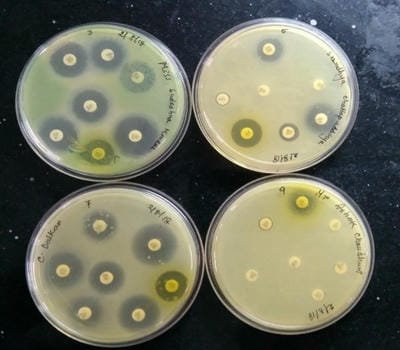
Introduction: Urinary tract infections (UTIs) are counted among the most common infections in humans. In spite of the availability and use of antimicrobial drugs, UTIs caused by bacteria have been showing increasing trends. The extensive and inappropriate use of antimicrobial agents has invariably resulted in the development of antibiotic resistance which, in recent years, has become a major problem worldwide. Materials and Methods: The study was conducted among the different ages of groups of both males and females attending KPC Medical College, Jadavpur, Kolkata during six months of the period included in this study. A total of 350 urine samples received from patients of the ICU and other wards of the medical college and hospital. All urine samples were processed within 1 hour after collection for aerobic bacterial culture. Results: Out of a total of 380 patients, isolates were detected in 350 (76.29%) samples. Out of these, 280 (80%) were female. The most common organism found positive was Escherichia Coli. E. coli was highly sensitive to Amikacin and Nitrofurantoin. Whereas, E.coli was highly resistant to Ampicillin and Nalidixic acid. The antibiotic sensitivity pattern of Klebsiella and Acinetobacter shows that they were also highly sensitive to Amikacin. Klebsiella and Acinetobacter were highly resistant to Ampicillin and Gentamicin. Conclusion: The pattern of resistance to commonly used antibiotics for treating UTI alerts us against indiscriminate usage of antibiotics.
Keywords: Urinary tract infection, Gram-negative, Antibiotic resistance
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Medicine, KPC Medical College and Hospital, Kolkata, West Bengal, India. Email: |
Mukherji T, Mukherji MB. Bacteriological profile and its antibiotic susceptibility in patients with urinary tract infection in tertiary care hospital. Trop J Pathol Microbiol. 2020;6(3):210-216. Available From https://pathology.medresearch.in/index.php/jopm/article/view/444 |


 ©
©