Cellular analysis of bronchoalveolar lavage fluid in various lung lesions
Punyashetty KB.1, Solanki PS.2*, Anand SA.3
DOI: https://doi.org/10.17511/jopm.2020.i02.09
1 Kajal B. Punyashetty, Professor, Department of Pathology, Navodaya Medical College, Raichur, Karnataka, India.
2* Padma Shree Solanki, Third-year Postgraduate student, Department of Pathology, Navodaya Medical College, Raichur, Karnataka, India.
3 Anand A. S., Professor and Head, Department of Pathology, Navodaya Medical College, Raichur, Karnataka, India.
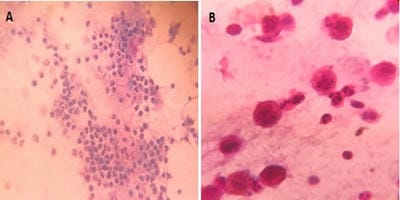
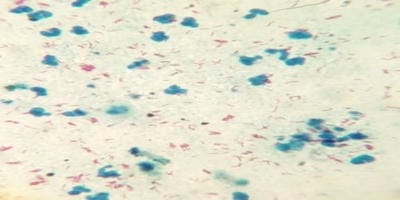
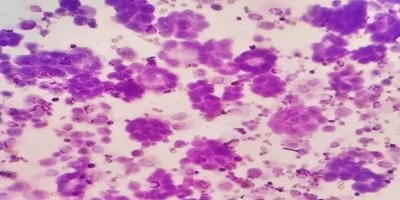
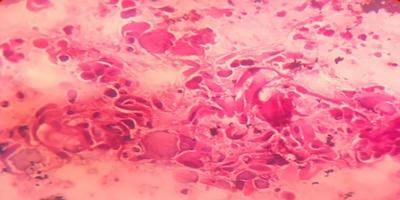
Introduction: Bronchoalveolar lavage (BAL) is a standard tool in the diagnosis of lung diseases. Cellular analysis of BAL fluid when deviates from normal are indicative of an inflammatory/infiltrative process. This study involves an analysis of BAL fluid performed manually and also using an automated analyzer which offers a rapid and reliable method for analysis of unprocessed BAL fluid. Aims and Objectives: To utilize the cellular analysis of bronchoalveolar lavage (BAL) fluid in the diagnosis of various lung diseases. Materials and Methods: 37 patients with pulmonary diseases of varying etiologies were included in this study. Fiberoptic bronchoscopy with BAL was performed. BAL fluid was run in SYSMEX XNL/350 six-part analyzer which gave total and differential cell count (mononuclear and polymorphonuclear cells). Results: Total WBC count ranged from 31 cells/µl to 94 cells/µl with the maximum increase seen in asthma. Mononuclear cells were increased in the majority of cases, being 84% in pneumoconiosis with the predominance of macrophages. An increase in lymphocytes was seen in Non-Specific Interstitial Pneumonia (45.3%) and Bronchiolitis Obliterans Organising Pneumonia (35.3%), neutrophils in Usual Interstitial Pneumonia (26.2%) and eosinophils in Chronic Eosinophilic Pneumonia (55.5%) and asthma (8.4%). Conclusion: BAL cell differentials when combined with additional clinical and radiographic information help secure a confident diagnosis in lung diseases and help clinicians to make therapeutic decisions.
Keywords: Automated six-part analyzer, Bronchoalveolar lavage fluid, Lung diseases
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Third-year Postgraduate student, Department of Pathology, Navodaya Medical College, Raichur, Karnataka, India. Email: |
Punyashetty KB, Solanki PS, Anand AS. Cellular analysis of bronchoalveolar lavage fluid in various lung lesions. Trop J Pathol Microbiol. 2020;6(2):167-173. Available From https://pathology.medresearch.in/index.php/jopm/article/view/437 |


 ©
©