A comparative study of conventional cytology and cell block method with immunohistochemistry in the diagnosis of serous effusions
Datta P.1, Saha R.2*, Chakraborty J.3
DOI: https://doi.org/10.17511/jopm.2020.i02.06
1 Pratyush Datta, Postgraduate trainee, Department of Pathology, ESI-PGIMSR, Manicktala, Kolkata, West Bengal, India.
2* Rama Saha, Senior Resident, Department of Pathology, ESI-PGIMSR, Manicktala, Kolkata, West Bengal, India.
3 Jayati Chakraborty, Professor, Professor and Head, ESI-PGIMSR, Manicktala, Kolkata, West Bengal, India.
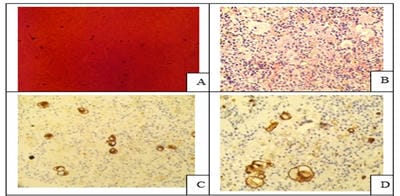
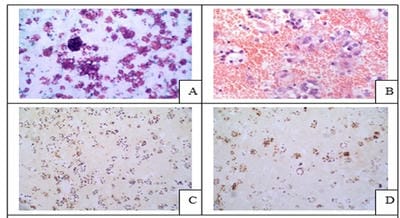
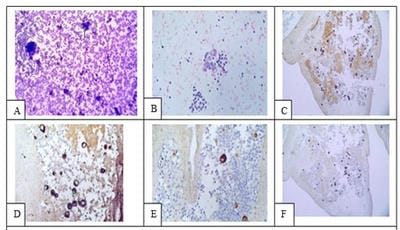
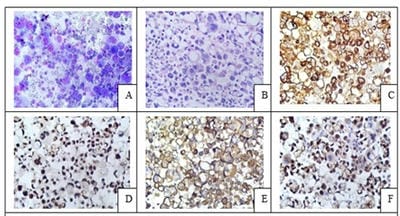
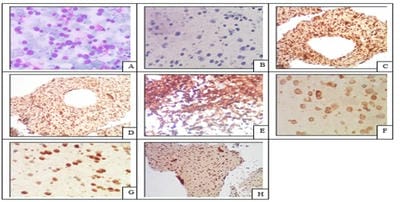
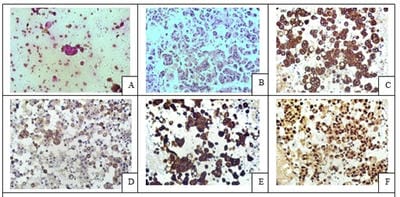
Introduction: Conventional smear (CS) examination of serous effusions is of paramount importance for diagnosis, staging, prognostication, and management of malignancy. The method has some disadvantages which can be overcome by cell block (CB) preparation. CB technique increases the diagnostic accuracy due to increased cellularity, preservation of tissue architecture and feasibility of performing immunohistochemistry (IHC). Aims and Objectives: To assess and compare the diagnostic yields of CS and CB techniques for detection of malignancy in pleural and peritoneal effusions and to study the utility of CB preparation with special emphasis on the feasibility of performing IHC in identifying the primary site of malignancy in the cases of carcinoma of unknown primary (CUP). Materials and Methods: In ESI-PGIMSR, Manicktala, each of 104 fluid samples were divided into two equal parts: one part was subjected to CS technique, the smears were stained with Leishman-Giemsa and Papanicolaou stains while the other part was subjected to Plasma thromboplastin CB technique and the sections stained by Hematoxylin and Eosin. IHC was performed whenever required. Provisional diagnoses made on CSs were compared with the diagnoses revised after examining CB slides. Results: Out of 104 fluid samples, on CS, 19 (18.27%) cases were positive for malignancy, whereas on CB 39(37.5%) cases were diagnosed as malignancy. The additional yield of malignancy was 19.23% more by the CB method. IHC done on CBs could suggest the possible primary site in 31 cases. Conclusions: The current study reports the diagnostic efficiency of the CB method to be superior to that of conventional smear alone as it has various advantages. Hence CB preparation should be routinely incorporated along with the use of IHC, if required, in the evaluation of serous effusions for a more accurate diagnosis.
Keywords: Cellblock, Conventional smear, Immunohistochemistry, Plasma Thromboplastin, Pleural and Peritoneal fluids
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Senior Resident, Department of Pathology, ESI-PGIMSR, Manicktala, Kolkata, West Bengal, India. Email: |
Datta P, Saha R, Chakraborty J. A comparative study of conventional cytology and cell block method with immunohistochemistry in the diagnosis of serous effusions. Trop J Pathol Microbiol. 2020;6(2):146-154. Available From https://pathology.medresearch.in/index.php/jopm/article/view/435 |


 ©
©