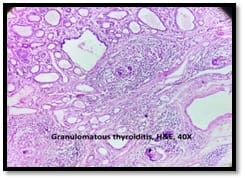
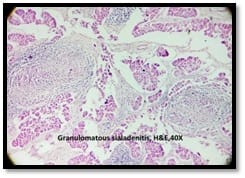
Granulomatous lesions !! Expected guests in unexpected places
Recharla M.1, Ramu R.2, Bindu J. B.3, Murthy C. N.4
DOI: https://doi.org/10.17511/jopm.2020.i04.02
1 Madhuri Recharla, Resident, Department of Pathology, Basaveshwara Medical College and Hospital, Rajiv Gandhi University of Health Sciences, Chitradurga, Karnataka, India.
2* Ramu R., Associate Professor, Department of Pathology, Basaveshwara Medical College and Hospital, Rajiv Gandhi University of Health Sciences, Chitradurga, Karnataka, India.
3 Bindu B. J., Associate Professor, Department of Pathology, Basaveshwara Medical College and Hospital, Rajiv Gandhi University of Health Sciences, Chitradurga, Karnataka, India.
4 Narayana Murthy C., Professor, Department of Pathology, Basaveshwara Medical College and Hospital, Rajiv Gandhi University of Health Sciences, Chitradurga, Karnataka, India.
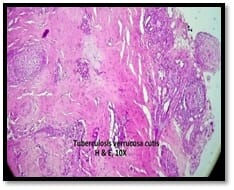
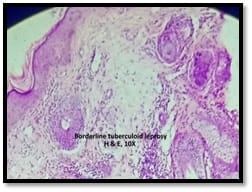
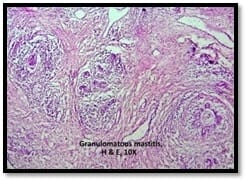
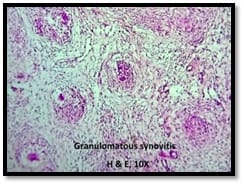
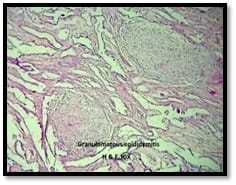
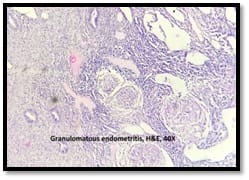
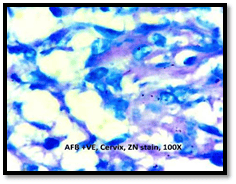
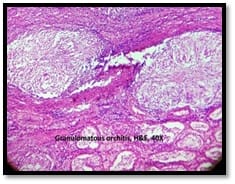
Introduction: Granulomatous lesions in various sites have different modes of presentation, different etiologic factors with identical histological patterns. The aim was to study the occurrence of granulomatous lesions at uncommon sites. Materials and methods: A retrospective study of histopathological specimens received over a period of one year from June 2017 to May 2018 was done and cases of granulomatous lesions of various sites reported on histopathological examination were reviewed along with Ziehl-Neelsen (ZN) and Fite-Faraco (FF) staining. Results: Out of total 3623 histopathology specimens, 61 (1.7%) were granulomatous lesions of which 28 (45.9%) were AFB (Acid-fast bacilli) positive. 20 cases (32.7%) were granulomatous lymphadenitis out of which 12 (19.6%) were positive for AFB. 4 (6.5%) out of 13 (21.3%) granulomatous skin lesions showed AFB positivity. 6 (9.8%) cases of granulomatous mastitis (1 was AFB +ve), 4(6.5%) cases of granulomatous synovitis (2 were AFB +ve), 3 (4.9%) cases of granulomatous endometritis (2 were AFB+ve) were seen. 2 (3.3%) cases each of granulomatous epididymitis and appendicitis were noted. Conclusion: In the present study tuberculosis was the most common cause of granulomatous lesions of various sites. Histopathology plays an important role in the diagnosis and management of a variety of granulomatous lesions. Special stains play a vital role in the diagnosis of infectious granulomatous lesions.
Keywords: Granulomatous lesions, Tuberculosis, Ziehl-Neelsen, Fite-Faraco
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pathology, Basaveshwara Medical College and Hospital, Rajiv Gandhi University of Health Sciences, Chitradurga, Karnataka, India. Email: |
Recharla M, Ramu R, Bindu BJ, Murthy CN. Granulomatous lesions !! Expected guests in unexpected places. Trop J Pathol Microbiol. 2020;6(4):308-317. Available From https://pathology.medresearch.in/index.php/jopm/article/view/430 |


 ©
©