Association of biofilm production in ESBL and MBL producing clinical isolates of Pseudomonas aeruginosa
Kulkarni D.M.1, Nilekar S.L.2, Vidhya T.3*
DOI: https://doi.org/10.17511/jopm.2020.i02.10
1 Kulkarni D.M., Associate Professor, Department of Microbiology, S.R.T.R Government Medical College, Ambajogai, Maharashtra, India.
2 Nilekar S.L., Professor and HOD, Department of Microbiology, S.R.T.R Government Medical College, Ambajogai, Maharashtra, India.
3* Vidhya T., Resident, Department of Microbiology, S.R.T.R Government Medical College, Ambajogai, Maharashtra, India.
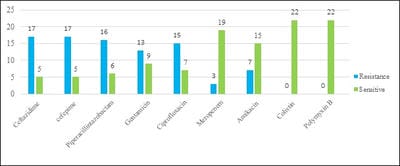
Introduction: Pseudomonas aeruginosa is one of the most prevalent nosocomial pathogens that cause a life-threatening infection. One of the important characteristics of P. aeruginosa is biofilm formation and the most studied bacterium related to biofilm formation so far. The biofilm formation and beta-lactamases production synergistically contribute to the extensive dissemination of multi-drug resistant strains. Aim: The present study was conducted to identify, biofilm-producing isolates of P. aeruginosa along with their antibiotic resistance pattern and ESBL and MBL production and to analyze their antibiogram. Materials and methods: Various clinical specimens were collected and totally 82 clinical isolates of P. aeruginosa were included in this study. Biofilm producing isolates were identified by the tube adherence method. Results: Among the total, 22 [26.83%] isolates were biofilm producers and the maximum number was obtained from blood [100%], followed by ETT [75%], and Drain [66.67%]. Biofilm producing isolates were showing more resistance in comparison to non-biofilm producers. Conclusion: High-level resistance to antimicrobial agents is a characteristic feature of infection caused by biofilm and lead to chronic infections. Knowledge about these biofilm-producing isolates is important in the clinical setting to eradicate these chronic and life-threating infections.
Keywords: Antibiogram, Biofilm, ESBL, MBL, Pseudomonas aeruginosa
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Resident, Department of Microbiology, S.R.T.R Government Medical College, Ambajogai, Maharashtra, India. Email: |
Kulkarni DM, Nilekar SL, Vidhya T. Association of biofilm production in ESBL and MBL producing clinical isolates of Pseudomonas aeruginosa. Trop J Pathol Microbiol. 2020;6(2):174-180. Available From https://pathology.medresearch.in/index.php/jopm/article/view/423 |


 ©
©