Uterine sarcomas: A clinicopathological analysis of 13 cases in a tertiary care centre
Pavithra P.1, Singh BK.2*, Singh VK.3, Kairanna NV.4, Rao N.5, Shetty T.6, Padmapriya J.7
DOI: https://doi.org/10.17511/jopm.2020.i02.05
1 Pavithra P., Associate Professor, Department of Pathology, Melaka Manipal Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
2* Brij Mohan Kumar Singh, Associate Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
3 Varun Kumar Singh, Assistant Professor, Department of Pathology, Melaka Manipal Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
4 Nikitha Valerina Kairanna, Assistant Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
5 Namrata Rao, Assistant Professor, Department of Pathology, Melaka Manipal Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
6 Tanvi Shetty, Associate Professor, Department of Pathology, Melaka Manipal Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
7 Padmapriya J., Associate Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
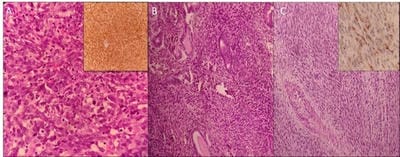
Introduction: Uterine sarcomas are rare tumors arising from mesodermal tissue of uterus which accounts for 1-3% of all female genital tract malignancies. They are characterized by rapid clinical progression and poor outcome. Materials and Methods: A retrospective review of clinical and pathological characteristics of patients with primary uterine sarcoma was done at Kasturba Hospital, Manipal between January 2013 and December 2018. Patients were staged using the 2018 FIGO histological classification for uterine cancer. Results: A total of 13 patients with uterine sarcoma were reviewed. All cases were multiparous women with the median age of presentation of 51.5 years (range: 40-80 years). Conclusion: Uterine sarcoma is an uncommon neoplasm with carcinosarcoma being the most common. The prognosis of uterine sarcomas depends on the histological subtype, grade and stage of the tumor at diagnosis.
Keywords: Adenosarcoma, Carcinosarcoma, Leiomyosarcoma, PEComa, Uterine sarcomas
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India. Email: |
Pavithra P, Singh BMK, Singh VK, Kairanna NV, Rao N, Shetty T, Padmapriya J. Uterine sarcomas: A clinicopathological analysis of 13 cases in a tertiary care centre. Trop J Pathol Microbiol. 2020;6(2):139-145.
Available From
https://pathology.medresearch .in/index.php/jopm/article/view/413 |


 ©
©