Diagnosis of primary site of tumour origin using immunohistochemistry on cell block in cases of malignant ascitic fluid
Patel TS.1, Goel DK.2*, Trivedi PP.3, Patel SP.4, Mehta SP.5
DOI: https://doi.org/10.17511/jopm.2020.i01.07
1 Trupti S. Patel, Associate Professor, Department of Pathology, Gujarat Cancer Research Institute, Ahmedabad, Gujarat, India.
2* Deepak K. Goel, Resident Doctor, Department of Pathology, Gujarat Cancer Research Institute, Ahmedabad, Gujarat, India.
3 Priti P. Trivedi, Professor and Head of Department, Department of Pathology, Gujarat Cancer Research Institute, Ahmedabad, Gujarat, India.
4 Suchita P. Patel, Senior Resident, Department of Pathology, Gujarat Cancer Research Institute, Ahmedabad, Gujarat, India.
5 Shailee P Mehta, Assistant Professor, Department of Pathology, Gujarat Cancer Research Institute, Ahmedabad, Gujarat, India.
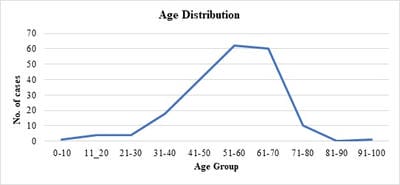
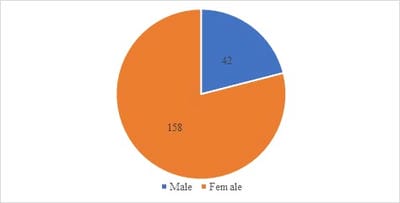
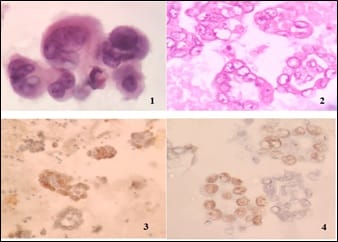
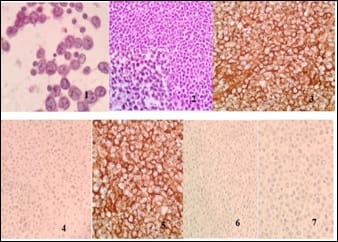
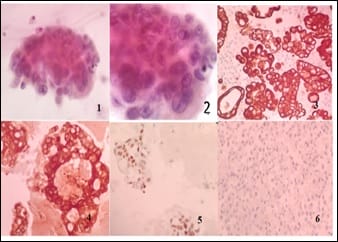
Introduction: Presence of malignant cells in peritoneal fluid has serious implications as most of the times, it represents a metastasis from a distant organ, along with poor prognosis. Aim: 1) To ascertain primary site of origin from malignant ascitic fluid cases using cytoblock preparation and immunohistochemistry. 2) To find out frequency of various cancers from different parts of the body in malignant peritoneal fluid. Materials and methods: This is retrospective cross-sectional observation-based study in which 200 cases of malignant peritoneal fluid were studied and analysed. Routine clinical data, radiological investigations, histopathological diagnosis, cytomorphological features and immunohistochemistry were considered and statistically analysed. Results: 1) Malignant ascitic fluid cases are more common in older age group of patients and females outnumber the male patients substantially. 2) Adenocarcinoma is the most common histological type. 3) Ovary is the most common primary site of malignant peritoneal effusion. 4) Cytoblock preparation and immunohistochemistry on cytoblock can be very helpful in finding the primary site of origin in those cases where patients first presented to the hospital with signs of malignant ascites and no symptomatology related to primary site. Conclusion: Cytological examination of serous effusions is an accurate, prompt, affordable technique having diagnostic and therapeutic implications. With the help of ancillary methods, the phenotype of cells can be identified, classify as well as confirm our diagnosis.
Keywords: Malignant ascites, Cell block, Immunohistochemistry, Primary site of origin
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Resident Doctor, Department of Pathology, Gujarat Cancer Research Institute, Ahmedabad, Gujarat, India. Email: |
Patel TS, Goel DS, Trivedi PP, Patel SP, Mehta SP. Diagnosis of primary site of tumour origin using immunohistochemistry on cell block in cases of malignant ascitic fluid. Trop J Pathol Microbiol. 2020;6(1):43-49. Available From https://pathology.medresearch.in/index.php/jopm/article/view/411 |


 ©
©