Testicular regression syndrome: A series of 22 cases at a tertiary care hospital
Sharma D.1, Sagar N.2*, Khurana N.3
DOI: https://doi.org/10.17511/jopm.2020.i02.12
1 Divya Sharma, Dr. Baba Saheb Ambedkar Medical College and Hospital, Delhi, India.
2* Nishant Sagar, Maulana Azad medical College and Hospital, Delhi, India.
3 Nita Khurana, Maulana Azad medical College and Hospital, Delhi, India.
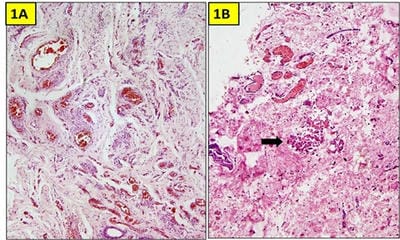
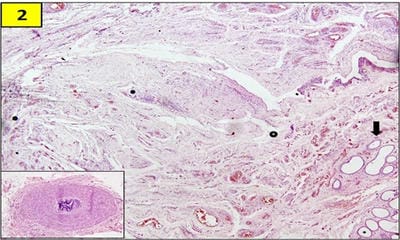
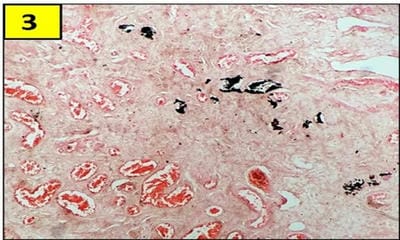
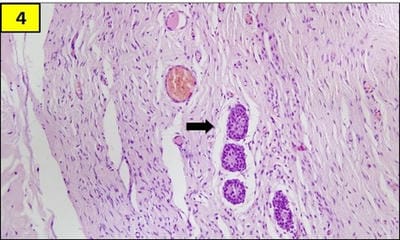
Testicular regression syndrome (TRS) represents a congenital condition in which no normal testicular tissue can be identified following exploration for a clinically impalpable testis. To study the Clinicopathological spectrum of Testicular regression syndrome (TRS) and review the literature. Study design: The study included 22 patients with nonpalpable testis, who had undergone resection of testicular nubbins. Original diagnosis was studied in context of pathological recognition of TRS and additional sections and stains were examined. Pathological assessment included identification of epididymis and vas deferens, vascularised fibrous nodule (VFN), dystrophic calcification, hemosiderin and pampiniform plexus like veins. Stain for iron and calcium were performed. On microscopy, VFN was observed in 17 (77.3%), calcification in 4 (18.2%), hemosiderin in 15 (68.2%), vas deferens in 13 (59%), epididymis in 11 (50%), prominent vessels in 21 (95.4%) and seminiferous tubules in 6 (27.3%) cases. The presence of dystrophic calcification and hemosiderin deposition with absent viable tissue points to the hypoxic injury to the testis. TRS theoretically carries a long-term risk for malignant degeneration therefore In the typical situation in which a blind-ending spermatic cord without viable testis is submitted for tissue analysis, it is imperative to characterize such cases as consistent with regressed testis thus eliminating the need for further surgical intervention.
Keywords: Impalpable, Regression, Vanishing testis, Nubbin
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Maulana Azad medical College and Hospital, Delhi, India. Email: |
Sharma D, Sagar N, Khurana N. Testicular regression syndrome: A series of 22 cases at a tertiary care hospital. Trop J Pathol Microbiol. 2020;6(2):191-197. Available From https://pathology.medresearch.in/index.php/jopm/article/view/406 |


 ©
©