Clinicopathological study of Meningioma
Patel JP.1, Jansari TR.2*, Chaudhari VV.3
DOI: https://doi.org/10.17511/jopm.2020.i01.02
1 Jigna Prakashbhai Patel, Assistant Professor, Department of Pathology, SBKS MI & RC, Sumandeep Vidyapeeth, Vadodara, Gujarat, India.
2* Trupti Rajeshbhai Jansari, Assistant Professor, Department of Pathology, SBKS MI & RC, Sumandeep Vidyapeeth, Vadodara, Gujarat, India.
3 Vaibhavi Vinodbhai Chaudhari, Resident Doctor, Department of Pathology, SBKS MI & RC, Sumandeep Vidyapeeth, Vadodara, Gujarat, India.
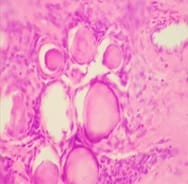
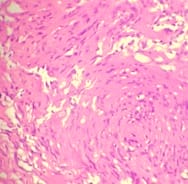
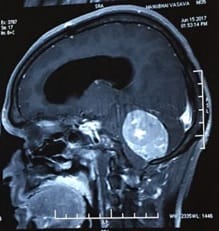
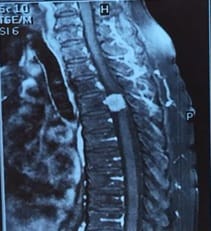
Introduction: Meningiomas are tumors that arise from the meningothelial cells. They are commonly located at intracranial, intraspinal or occasionally ectopic site. They show histological diversity and are categorized into three grades. Aims and Objectives: To study the incidence, anatomical location, sex and age Predilection, histological variants and grading of meningiomas based on WHO 2016 classification. To correlate clinical features and radiological findings with those of histopathological findings. Materials and Methods: The study is carried out in the Department of Pathology, Dhiraj General Hospital, Piparia from November 2016 to July 2018. 30 tumors specimen diagnosed as meningioma by radiology and neurosurgery department, sent to department of pathology were included in the study. Results: Total 30 meningioma tumors were included in the study. Most of them were intracranial, predominantly involving the posterior fossa of brain, females and the 41-60 age group. The most common histological subtype was psammomatous followed by meningothelial. Majority (93.33%) were benign grade I tumors. Conclusion: Meningiomas are slow growing tumors arising from the meningothelial cells accounting for 15-30 % of all CNS neoplasms showing a variety of histological patterns, more common in women, predominantly Grade I tumors.
Keywords: Intracranial, Meningioma, Meningothelial cells, WHO grade
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Pathology, SBKS MI & RC, Sumandeep Vidyapeeth, Vadodara, Gujarat, India. Email: |
Patel PJ, Trupti RJ, Chaudhari VV. Clinicopathological study of Meningioma. Trop J Pathol Microbiol. 2019;6(1):9-17. Available From https://pathology.medresearch.in/index.php/jopm/article/view/403 |


 ©
©