Study of haematological parameters in malaria: a prospective study
Babariya MJ.1, Parmar JS.2*
DOI: https://doi.org/10.17511/jopm.2020.i01.04
1 Manjula J Babariya, Assistant Professor, Department of Microbiology, Banas Medical College and Research Institute, Palanpur, Gujarat, India.
2* Jitendrakumar S. Parmar, Assistant Professor, Department of Pathology, Banas medical College and Research Institute, Palanpur, Gujarat, India.
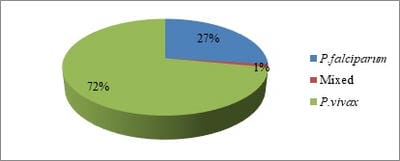
Background: A blood parasites plasmodia is responsible for malaria hence there are haematological alterations in malaria. The haematological changes that have been reported to accompany malaria include anaemia, thrombocytopenia, leucocytosis and leucopenia. Methods: Total 585 smear positive malaria cases were taken and various haematological parameters were studied. Results: Out of 585 smear positive cases, P. vivax was positive in 422 (72%) cases, while P. falciparum was positive in 160 (27%) cases and mixed infection was found in 3 (1%) cases. Anaemia was seen in 420 (71.79%) cases. Normocytic normochromic blood picture was the most common type in anaemic patients 223 (38.11%). Next common finding was Normocytic hypochromic RBCs 185 (31.62%). Microcytic hypochromic RBCs found in 152 (25.98%) cases. Thrombocytopenia was seen in total 490 (83.76%) of the patients. Moderate thrombocytopenia was more common and present in 409 (70%) of patients while Severe thrombocytopenia was seen in 81 (13.84%) of cases. Conclusions: Various haematological findings can help in early diagnosis of malaria which is essential for timely and appropriate treatment which can limit the morbidity and prevent further complications.
Keywords: CBC, Haematological-parameters, Malaria, Thrombocytopenia
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Pathology, Banas medical College and Research Institute, Palanpur, Gujarat, India. Email: |
Manjula J Babariya, Jitendrakumar S. Parmar, Study of haematological parameters in malaria: a prospective study. Trop J Pathol Microbiol. 2020;6(1):23-29. Available From https://pathology.medresearch.in/index.php/jopm/article/view/384 |


 ©
©