Spectrum of IgA nephropathy in a tertiary care centre, Chennai
Gomathi R.1*
DOI: https://doi.org/10.17511/jopm.2019.i08.11
1* Gomathi R., Shri Sathya Sai Medical College Sri Balaji Vidhyapeeth University, Puducherry, Tamil Nadu, India.
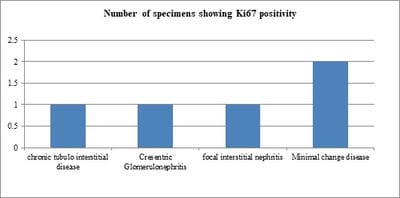
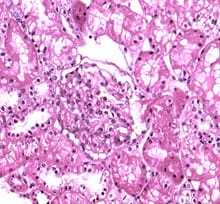
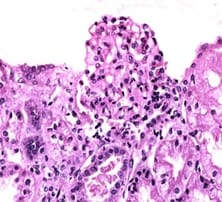
Background: IgA nephropathy is a common renal phenomenon which progresses to chronic kidney disease. It accounts for approximately one-third of the total renal biopsy diagnosis and nearly one half of all primary glomerular diseases diagnosed by renal biopsy. Ki67 functions as a nuclear marker and measures the proliferation index which is seen as increase in the proliferation in glomeruli and also in proximal tubules and renal interstitium. There is a great degree of histologic variability in this disease, varying from minimal histological lesion to a diffuse proliferative nephritis and glomerulosclerosis. This study was carried out to evaluate the spectrum of IgA nephropathy with Ki67 expression. Methods: This cross-sectional record-based study was carried out on 16 renal core biopsies. The cases were histologically diagnosed as IgA nephropathy. Hematoxylin and Eosin staining was done on the slides and immunohisto chemistry was done on paraffin blocks for evaluation of Ki67 using mouse antihuman Ki67 monoclonal antibody-Biogenex at the dilution of 1:50. Results: The most common histological variant was focal proliferative glomerulonephritis Haas Class III. Minimal change disease and focal segmental glomerulosclerosis were the most prevalent histological subtypes. It was observed that chronic tubulointerstitial disease, cresentric glomerulonephritis, Minimal Change Disease and focal interstitial nephritis were positive of Ki67 in 5 cases (31%). Conclusion: Although this study was undertaken on a relatively small number of specimens, the preliminary data obtained from this study could be the basis for a future prospective analysis in a larger number of cases of IgAN in order to be statistically significant.
Keywords: IgA nephropathy, Ki-67, Immunohistochemistry, Chronic kidney disease
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Shri Sathya Sai Medical College Sri Balaji Vidhyapeeth University, Puducherry, Tamil Nadu, India. Email: |
Gomathi R. Spectrum of IgA nephropathy in a tertiary care centre, Chennai. Trop J Pathol Microbiol. 2019;5(8):574-579. Available From https://pathology.medresearch.in/index.php/jopm/article/view/305 |


 ©
©