Prevalence of acute undifferentiated febrile illnesses in a tertiary care centre in Telangana, South India
Mohan M.1*, Teja V.2, Subhada K.3
DOI: https://doi.org/10.17511/jopm.2019.i08.08
1* M. V. N. L. Ram Mohan, Assistant Professor, , Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
2 V.D. Teja, Professor & Head, , Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
3 Subhada K, Lab Investigator, Department of Microbiology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
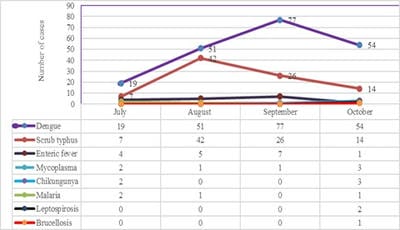
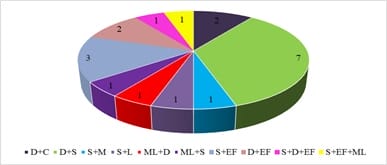
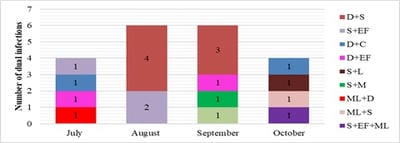
Introduction: Increased incidence of acute undifferentiated febrile illnesses (AUFI) are observed with the beginning of monsoon season in tropical countries. Diverse aetiologies, overlapping clinical presentations and mixed infections complicate the diagnosis and management of febrile illnesses. Knowledge of the local aetiology and seasonal prevalence of these diseases would enable physicians and policy makers to take adequate control measures. Hence the present study was undertaken to understand the aetiology of AUFI and the prevalence of multiple infections in patients attending our tertiary care hospital in Telangana, South India. Material and Methods: A total of 932 patients presenting with AUFI over a period of four months from July to October 2018 were tested for various causes of fever by serological tests. Dengue, scrub typhus, Leptospira and Chikungunya were tested by rapid tests and ELISA. Latex agglutination kits were used for diagnosis of Salmonella and Brucella infections. Peripheral smear examination was used to diagnose malaria. Results: Dengue was the most common infection seen in 21.6% of patients followed by scrub typhus in 9.5%. Peak incidence of Dengue was seen in the month of September and maximum scrub typhus cases were diagnosed in August. Dual infections were documented in 1.9% of patients; most common being dengue with scrub typhus. Conclusion: Awareness of the local aetiology of AUFI guides clinicians in prioritising clinical and diagnostic workup and initiating appropriate empirical and supportive therapy. As incidence of multiple infections is increasing, comprehensive clinical and diagnostic exploration for probable pathogens need to be considered in treatment non-responsive AUFI patients.
Keywords: AUFI, Dual infections, Dengue, Scrub typhus, Seasonal prevalence
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India. Email: |
M V N L Ram Mohan, VD Teja, Subhada K. Prevalence of acute undifferentiated febrile illnesses in a tertiary care centre in Telangana, South India. Trop J Pathol Microbiol. 2019;5(8):555-561. Available From https://pathology.medresearch.in/index.php/jopm/article/view/304 |


 ©
©