Synchronous primary cancer of the thyroid and breast
Rakshitha H.M1*, Suma M.N.2, Arjunan R.3, Malathi M.4, Priya D.5, Premalata C.S.6
DOI: https://doi.org/10.17511/jopm.2019.i07.08
1* Rakshitha H.M., Department of Pathology, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
2 Suma M.N., Department of Pathology, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
3 Ravi Arjunan, Department of Surgical Oncology, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
4 Malathi M, Department of Pathology, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
5 Priya D., Department of Pathology, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
6 Premalata C.S., Department of Pathology, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
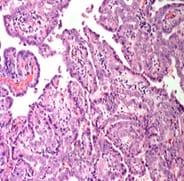
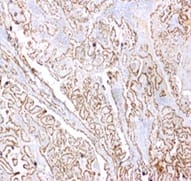
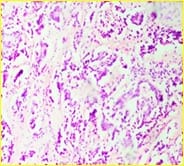
Background: The present case is of a synchronous primary classical thyroid carcinoma and primary ductal carcinoma of the breast in a 57 year old lady. Though the incidence of both thyroid cancer and breast cancer have been rising in recent years, it is very rare to find a single person with both of these cancers. Case summary: Patient underwent total thyroidectomy and right breast lump excision. Post operative histopathological examination identified papillary carcinoma thyroid, classical type, of the right lobe with metastasis to central compartment lymph nodes and invasive ductal carcinoma of the right breast. Now the patient is on regular follow up. Thus the aim of the current report was to improve the understanding of synchronous primary tumors of the thyroid and breast by presenting a review of the associated literature regarding breast and thyroid carcinoma. Conclusion: Although synchronous primary tumors of the thyroid and breast are very rare, they remain a possibility, therefore more attention should be paid to this cancer.
Keywords: Synchronous, FNAC, Papillary thyroid
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Department of Pathology, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India. Email: |
Rakshitha HM, Suma MN, Arjunan R, Malathi M, Priya D, Premalata CS. Synchronous primary cancer of the thyroid and breast. Trop J Pathol Microbiol. 2019;5(7):461-465. Available From https://pathology.medresearch.in/index.php/jopm/article/view/296 |


 ©
© 
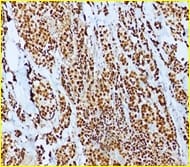
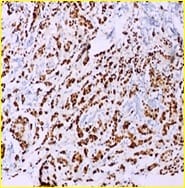 F.
F.