Expression of EGFR in non small cell lung carcinoma
Devi E. P.1*, Sai Shalini C.N.2, Prathiba D.3
DOI: https://doi.org/10.17511/jopm.2019.i07.12
1* Prema Devi E., Assistant Professor, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
2 Sai Shalini C.N., Associate Professor, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
3 Prathiba D., Professor, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
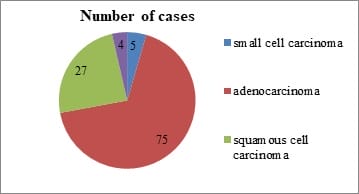
Background: The incidence and mortality associated with lung cancers are increasing at an alarming rate. Studies have shown that activation of Epidermal Growth Factor Receptor (EGFR) triggers the tumorigenesis in these cancers. The potential role of analyzing EGFR expression in these carcinomas could go a long way in devising screening tools for early detection of lung carcinoma. This study was carried out to evaluate the prevalence and factors associated with EGFR expression. Methods: This cross sectional study was carried out among 75 paraffin block specimens of lung carcinoma received in our tertiary care center for a period of five years. Three micron thick paraffin sections were cut and Hemotoxylin & Eosin staining was done. All the adenocarcinoma cases were immunostained with EGFR antibody and the results were analyzed. Results: Majority of the tumors were moderately differentiated (46%) and were negative for lymph node metastasis (69%). With regards to EGFR positivity, majority of the tumors showed EGFR expression 3+ (57.3%) flowed by 2+ (16%). Among the EGFR negative cases, ALK expression was positive in 5% of the cases. Conclusion: The present study has reinforced the fact that Indian patients have high expression of EGFR and therefore can be benefitted by targeted therapy. IHC coupled with molecular analysis would be of maximum benefit to patients with EGFR & ALK mutations.
Keywords: EGFR, Immunohistochemistry, Lung cancer, Tyrosine kinase
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, , Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India. Email: |
Devi EP, Sai Shalini CN, Prathiba D. Expression of EGFR in non small cell lung carcinoma. Trop J Pathol Microbiol. 2019;5(7):484-492. Available From https://pathology.medresearch.in/index.php/jopm/article/view/292 |


 ©
©