Histomorphological study of uterine leiomyomas and its variants with brief review of literature
Budihal N.1, G. Pawar J.2, Manasa G. C.3*
DOI: https://doi.org/10.17511/jopm.2019.i07.09
1 Nischita Budihal, Assistant Professor, Department of Pathology, Sri Siddhartha Institute of Medical Sciences and Research Centre, Nelamangala, Karnataka, India.
2 Jayashree G. Pawar, Professor, BLDE Medical College, Vijayapura, Karnataka, India.
3* Manasa G. C., Associate Professor, JJM Medical College, Davangere, Karnataka, India.
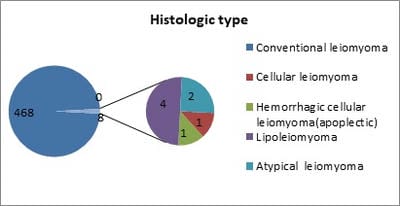
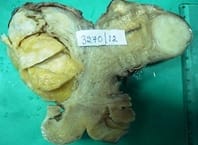
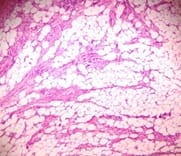
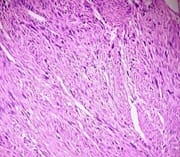
Background: Uterine mesenchymal tumours are a heterogeneous group of neoplasms that can frequently be diagnostically challenging. Most subtypes of leiomyoma are chiefly of interest in that they mimic malignancy in one or more respects. Objective: To evaluate the histomorphological features of uterine leiomyomas and its variants. Materials and methods: Total of 477 cases of uterine leiomyomas and its variants were analysed prospectively in a period of 2years during July 2010 to June 2012 to assess the various pattern of leiomyomas. Cases were studied in detail about complete history, clinical examination and other findings. Results: In the study 468 (98.11%) cases showed features of conventional leiomyoma and 8 cases showed variants of leiomyomas (1.68%). Conclusion: Although their diagnosis is straight forward in most cases, difficulties arise with particular leiomyoma variants, especially highly cellular leiomyoma (often confused with an endometrial stromal tumour) and leiomyoma with bizarre nuclei, mitotically active leiomyoma which may cause concern for leiomyosarcoma.
Keywords: Apoplectic, Cellular, Leiomyoma, Lipoleiomyoma, Uterus, Variants
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, JJM Medical College, Davangere, Karnataka, India. Email: |
Budihal N, Pawar JG, Manasa GC. Histomorphological study of uterine leiomyomas and its variants with brief review of literature. Trop J Pathol Microbiol. 2019;5(7):466-472. Available From https://pathology.medresearch.in/index.php/jopm/article/view/289 |


 ©
©