A study on evaluation of Escherichia coli isolates in urinary tract infection and its antibiogram in view of Emerging drug resistance at a tertiary care hospital
Wajid M.1*, Mallamgunta S.2, Pedapati V.3, Naaz S.4
DOI: https://doi.org/10.17511/jopm.2021.i06.01
1* Mahamad Wajid, Associate Professor, Department of Microbiology, ESIC Medical College and Hospital, Sanathnagar, Hyderabad, India.
2 Saranya Mallamgunta, Assistant Professor, Department of Microbiology, ESIC Medical College and Hospital, Sanathnagar, Hyderabad, India.
3 V. Prathyusha Pedapati, Senior Resident, , ESIC Medical College and Hospital, Sanathnagar, Hyderabad, India.
4 Shazia Naaz, Assistant Professor, Department of Microbiology, ESIC Medical College and Hospital, Sanathnagar, Hyderabad, India.
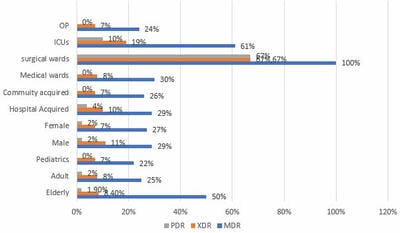
Introduction: Urinary tract infection is a common problem in routine clinical practices despite age and sex, and it is also the most common nosocomial infection. The most common uropathogen is Escherichia coli worldwide. Material and methods: A retrospective study was conducted from January 2020 to December 2020 in a tertiary-care hospital in South India. All positive urinary cultures with significant bacteriuria received from Inpatient and outpatient wards of various departments were included in the study. The prevalence percentage of different bacteria was studied. Multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan drug-resistant (PDR) Escherichia coli in different baseline and demographic characteristics were evaluated. Results: A total of 2144 urine samples were processed for culture and sensitivity, out of which 531 samples were found to be culture-positive with significant bacteuria. Among 531 samples, 202 (38%) culture positives were due to Escherichia coli infection. Women (62%) were more susceptible to infection. The predominant age group affected among women was 21-30 yrs and men 51-60 years. Of these, 28% exhibited an MDR pattern, 18% XDR and 1.9% PDR. Conclusion: Treatment of Urinary tract infection due to E. coli may be difficult due to high resistance to commonly used antibiotics. So continuous surveillance of drug-resistant organisms and evaluation of drug resistance patterns are needed to prevent treatment failure and to reduce the economic burden due to these resistant isolates.
Keywords: Escherichia coli, Multidrug resistance, Community-acquired, Hospital-acquired
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Microbiology, ESIC Medical College and Hospital, Sanathnagar, Hyderabad, India. Email: |
Mahamad Wajid, Saranya Mallamgunta, V. Prathyusha Pedapati, Shazia Naaz, A study on evaluation of Escherichia coli isolates in urinary tract infection and its antibiogram in view of Emerging drug resistance at a tertiary care hospital. Trop J Pathol Microbiol. 2021;7(6):272-279. Available From https://pathology.medresearch.in/index.php/jopm/article/view/587 |


 ©
©