Solid Pseudopapillary Neoplasm of the Pancreas - A rare entity with emphasis on the differential diagnosis.
Khan S.1*, Trisal M.2, Husain M.3, Khetrapal S.4, Hassan M.5, Jetley S.6
DOI: https://doi.org/10.17511/jopm.2021.i01.10
1* Sabina Khan, Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, New Delhi, India.
2 Monal Trisal, Demonstrator, Department of Pathology, Hamdard Institute of Medical Sciences and Research, New Delhi, India.
3 Musharraf Husain, Professor and Head of Department, Department of Surgery, Hamdard Institute of Medical Sciences and Research, New Delhi, India.
4 Shaan Khetrapal, Assistant Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, New Delhi, India.
5 Mohd. Jaseem Hassan, Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, New Delhi, India.
6 Sujata Jetley, Professor and Head of Department, Department of Pathology, Hamdard Institute of Medical Sciences and Research, New Delhi, India.
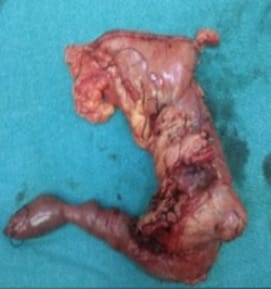
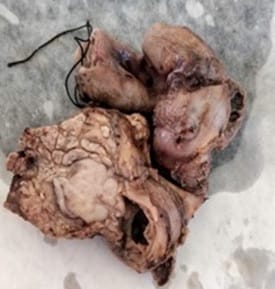
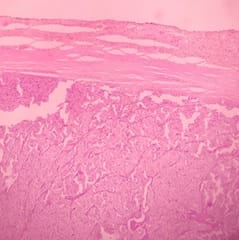
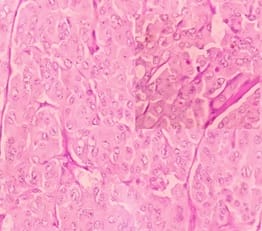
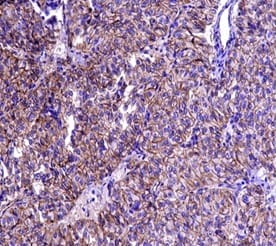
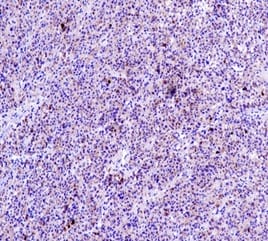
Solid Pseudopapillary Neoplasm of Pancreas (SPNP) is a rare, low-grade malignant solid-cystic neoplasm with papillary architecture. It accounts for 2% to 3% of pancreatic neoplasms and 0.9% to 2.7% of exocrine pancreatic neoplasms. It occurs almost exclusively in young women and has an excellent postsurgical curative rate. Metastasis is rare although it may be locally aggressive. The solid pseudopapillary neoplasm of the pancreas pose a diagnostic challenge both clinically and radiologically as it has a nonspecific clinical presentation with vague radiologic features. Histopathological evaluation and immunohistochemistry remains the gold standard in reaching a definitive diagnosis. Due to its low incidence, the clinical and pathologic features of SPNP have not been extensively studied. We report a case of a 32-year-old lady with solid pseudopapillary neoplasm of the pancreas that was suspected on abdominal CECT as a well-defined mass in the ampullary-periampullary region abutting head of the pancreas and confirmed on histopathological evaluation with immunohistochemistry.
Keywords: Pancreatic tumours, Exocrine tumours of pancreas, Solid pseudopapillary neoplasm
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, New Delhi, India. Email: |
Khan S, Trisal M, Husain M, Khetrapal S, Hassan J, Jetley S. Solid Pseudopapillary Neoplasm of the Pancreas - A rare entity with emphasis on the differential diagnosis.. Trop J Pathol Microbiol. 2021;7(1):60-64. Available From https://pathology.medresearch.in/index.php/jopm/article/view/512 |


 ©
©