Analysis of diagnostic value of cytological smear method versus cell block method in body fluids with clinical and biochemical correlation: study of 150 cases
H. Chandan R.1, Pawar S.2*, Reddy P.3
DOI: https://doi.org/10.17511/jopm.2021.i01.02
1 Rajesh H. Chandan, Professor, Department of Pathology, Karnataka Institute of Medical Sciences, Hubballi, Karnataka, India.
2* Sumana Pawar, Post graduate student, Department of Pathology, Karnataka Institute of Medical Sciences, Hubballi, Karnataka, India.
3 Purushotham Reddy, Professor and Head of the department, Department of Pathology, Karnataka Institute of Medical Sciences, Hubballi, Karnataka, India.
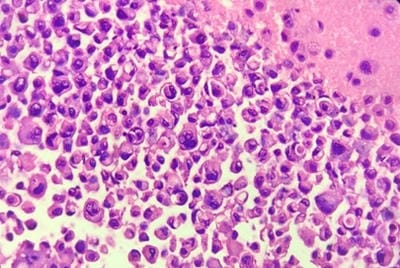
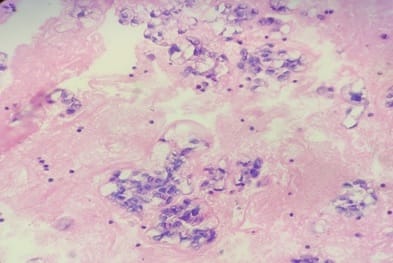
Background: Aspiration of serous cavities is a simple and relatively non-invasive technique to achieve diagnosis. Cytological evaluation of body cavity fluid is diagnostically challenging. Especially in malignant effusions, helps in staging, prognosis and management of the patients. Aims: To assess the utility and sensitivity of cell block method over conventional smear technique in cytodiagnosis of the serous effusions. And to assess the utility and sensitivity of cytological evaluation of body fluids with biochemical and clinical correlation. Methods: A total of 150 fluid specimens were examined for conventional cytological smear (CS) and cell block method (CB). Out of 150 fluids, 96 were pleural fluid, 48 were ascitic fluid, 04 fluid from pouch of Douglas and 01 was from synovial fluid. Results: In this study, the utility of the CB method in the cytodiagnosis of malignant effusions was found to be highly significant as compared to the CS method. The additional yield of malignancy was 12% more as was obtained by the CB method. Conclusion: For the final cytodiagnosis of body fluid, there is statistically significant difference between the two techniques. In other words, CB is superior to CS method. It gives more information about the architectural arrangement and the likely source of primary. More important is that diagnostic material in cell blocks is available for special studies for Immunohistochemistry which can further supplement our knowledge about the primary source of metastasis.
Keywords: Cell block, Conventional Smear, Cytodiagnosis, Effusion
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Post graduate student, Department of Pathology, Karnataka Institute of Medical Sciences, Hubballi, Karnataka, India. Email: |
Chandan RH, Pawar S, Reddy P. Analysis of diagnostic value of cytological smear method versus cell block method in body fluids with clinical and biochemical correlation: study of 150 cases. Trop J Pathol Microbiol. 2021;7(1):9-16. Available From https://pathology.medresearch.in/index.php/jopm/article/view/492 |


 ©
©