Conventional transbronchial needle aspiration cytology as a diagnostic tool in patients with suspected lung cancer - Our experience at a tertiary care center.
Chauhan R.1, Awasthi S.2, Arora D.3*, Ahmed F.4, Joshi H.5, Garg I. 6
DOI: https://doi.org/10.17511/jopm.2020.i07.01
1 Rashmi Chauhan, Assistant Professor, 2 Seema Awasthi, Professor and Head, 3* Deepti Arora, Associate Professor, 4 Faiyaz Ahmed, Professor, 5 Himanshu Joshi, Associate Professor, 6 Ina Garg, Post-Graduate. All the authors are affiliated with the Department of Pathology, Teerthanker Mahaveer Medical College and Research Center, Moradabad, Uttar Pradesh, India
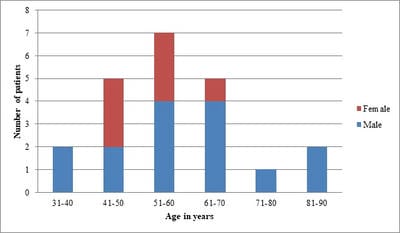
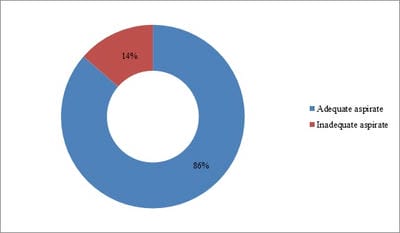
Background: Conventional transbronchial needle aspiration (c-TBNA) is a minimally invasive bronchoscopic technique used to obtain cytological samples from peribronchial lesions and mediastinal lymph nodes. However, the concern about its efficacy and the advent of newer techniques have led to the underutilization of this time tested and cost-effective modality. Objective: The present study was aimed to assess the diagnostic yield of c-TBNA in suspected cases of lung cancer. Method: c-TBNA smears received from January 2017 to February 2020, with clinical-radiological suspicion of lung malignancy were retrospectively analyzed. Result: A total of 22 cases were reviewed. The mean age of the study population was 57.54 years, with a male-female ratio of ~2:1. The adequate aspirate was obtained in 19/22 (86%) cases. The overall diagnostic yield of c-TBNA was 82%. 14/19 (74%) cases were positive for malignancy, non-small cell lung carcinoma being the most common malignancy diagnosed (11 cases). 4/19 (21%) cases were diagnosed with granulomatous pathology, while smears in 1 case were non-diagnostic. Conclusion: Conventional transbronchial needle aspiration cytology is an efficacious method used for the diagnosis of lung carcinoma. Especially in resource-limited settings, it remains irreplaceable as a diagnostic tool and should be routinely utilized.
Keywords: Bronchoscopy, Lung cancer, Transbronchial needle aspiration
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pathology, Teerthanker Mahaveer Medical College and Research Center, Moradabad, Uttar Pradesh, India. Email: |
Chauhan R, Awasthi S, Arora D, Ahmed F, Joshi H, Garg I. Conventional transbronchial needle aspiration cytology as a diagnostic tool in patients with suspected lung cancer - Our experience at a tertiary care center.. Trop J Pathol Microbiol. 2020;6(7):417-424. Available From https://pathology.medresearch.in/index.php/jopm/article/view/479 |


 ©
©