Mural nodule of Anaplastic Carcinoma in an Ovarian Mucinous Cystadenoma- A rare case report
Subashree K.1, Lavanya M.2*, Jayanthi3, Maria4
DOI: https://doi.org/10.17511/jopm.2020.i04.13
1 Subashree K., Assistant Professor, Department of Pathology, Sri Venkateshwaraa Medical College and Research Centre, Pondicherry, India.
2* Lavanya M, Associate Professor, Department of Pathology, Sri Venkateshwaraa Medical College and Research Centre, Pondicherry, India.
3 Jayanthi, Assistant Professor, Department of Pathology, Sri Manakula Vinayakar Medical College, Pondicherry, India.
4 Maria, Postgraduate, Department of Pathology, Sri Venkateshwaraa Medical College and Research Centre, Pondicherry, India.
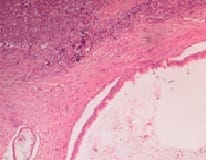
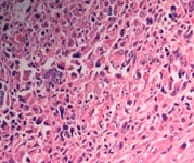
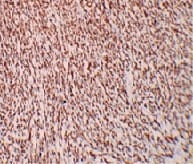
Mucinous tumors account approximately for 15% of all primary ovarian epithelial neoplasms. Ovarian mucinous tumors with mural nodules are rare surface epithelial-stromal tumorswhich can be of three types, "sarcoma-like"?, sarcoma or anaplastic carcinoma. Mural nodules of anaplastic carcinoma were first described in 1982 by Prat et al following which approximately fifty cases have been reported to date and in those cases, only one has been associated with benign mucinous tumors. The current case report is from a 50-year-oldpostmenopausal woman who presented to the gynecology outpatient department with complaints of abdominal pain and distension for the past five months. On histopathological examination, a diagnosis of Mucinous cystadenoma with focal atypia and mural nodule of anaplastic carcinoma was made and confirmed with immunohistochemistry.
Keywords: Anaplastic carcinoma, Ovarian mucinous neoplasms, Mural nodules
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pathology, Sri Venkateshwaraa Medical College and Research Centre, Pondicherry, India. Email: |
Subashree K, Lavanya M, Jayanthi, Maria. Mural nodule of Anaplastic Carcinoma in an Ovarian Mucinous Cystadenoma- A rare case report. Trop J Pathol Microbiol. 2020;6(4):341-344. Available From https://pathology.medresearch.in/index.php/jopm/article/view/464 |


 ©
©