Correlation of ER, PR, Her2neu and Ki67 with other Prognostic factors in Breast Carcinoma
Reddy P.1, Mithraa DS.2*
DOI: https://doi.org/10.17511/jopm.2020.i05.01
1 Purushotham Reddy, Professor and HOD, Department of Pathology, Karnataka Institute of Medical Sciences, Hubballi, Karnataka, India.
2* Mithraa Devi S, Consultant Pathologist, Hitech Diagnostic Centre, Chennai, Tamil Nadu, India.
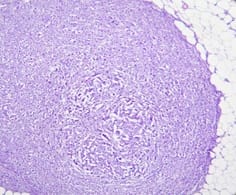
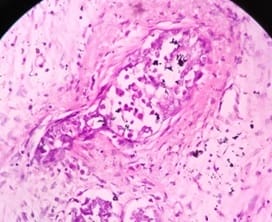
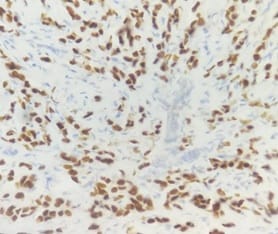
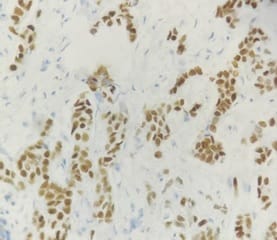
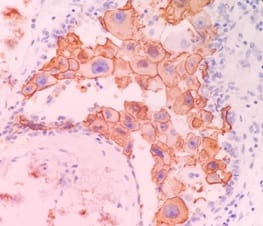
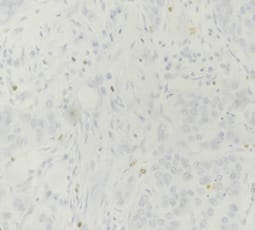
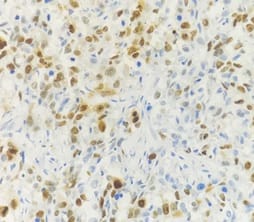
Background: In females, breast carcinoma is the most common malignancy accounting for 23% of all malignant tumours. Various predictive and prognostic factors affect tumour progression. In addition to estrogen receptors (ER), progesterone receptors (PR) and human epidermal growth factor receptor (Her2neu), recently Ki67, a proliferative marker has been recognized as an important predictive and prognostic marker in many studies. The aim of the study was to find the correlation of ER, PR, Her2neu and Ki67 tumour markers with menopausal status, tumour size, histopathological grade, mitotic index, lymphovascular invasion and lymph node metastasis. Methods: The present study was conducted on 50 cases of breast carcinomas. The histopathological grading of the breast carcinoma was done according to the Nottingham modification of the Bloom Richardson grading system. All the cases underwent immunohistochemistry for ER, PR, Her2neu and Ki67 expression. Correlation of ER, PR, Her2neu and Ki67 with various prognostic factors was done. Results: The expression of ER and PR decreased as the grade of the tumour increased. Ki67 proliferative index increased as the grade of the tumour increased. Ki67 proliferative index also increased as the mitotic count increased. None of these markers showed a correlation with other prognostic factors. Conclusion: The present study concludes that ER, PR reveals inverse relationship and Ki67 showed a direct relationship with the grade of the tumour.
Keywords: Breast Carcinoma, Estrogen receptors, Progesterone receptors, Human epidermal growth factor receptor, Ki67
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Consultant Pathologist, Hitech Diagnostic Centre, Chennai, Tamil Nadu, India. Email: |
Reddy P, Mithraa SD. Correlation of ER, PR, Her2neu and Ki67 with other Prognostic factors in Breast Carcinoma. Trop J Pathol Microbiol. 2020;6(5):349-361. Available From https://pathology.medresearch.in/index.php/jopm/article/view/459 |


 ©
©