Incidence, microbiological profile and outcome of ventilator-associated events in a tertiary care hospital in Bangalore, Karnataka, India
Shashikala N.1, Manasa S.2*, Hedge D.3, Vikram V.4, Kumar P M.5
DOI: https://doi.org/10.17511/jopm.2020.i02.04
1 Shashikala N, Infection Control Officer, Department of Pathology, Sagar Hospital, Bangalore, Karnataka, India.
2* Manasa S, Junior Infection Control Officer, Department of Pathology, Sagar Hospital, Bangalore, Karnataka, India.
3 Deviprasad Hedge, Head of the Department (ICU), Department of Pathology, Sagar Hospital, Bangalore, Karnataka, India.
4 Venkatesh Vikram, Medical Director, Department of Pathology, Sagar Hospital, DSI, Bangalore, Karnataka, India.
5 Mahendra Kumar P, Medical Director, Department of Pathology, Sagar Hospital, Bangalore, Karnataka, India.
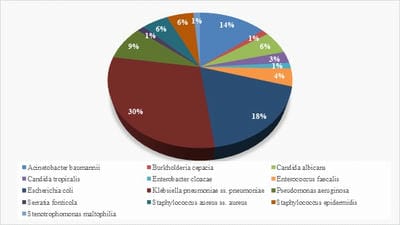
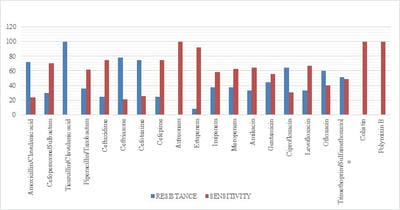
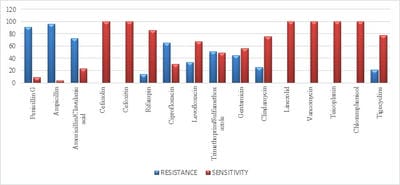
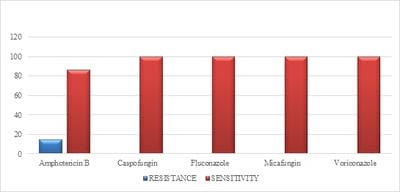
Introduction: Despite different ICU admission causes, ventilator-associated event (VAE) is still a common cause of mortality and morbidity in intubated patients and impedes obvious progression in diagnostic modalities and management of these infections. Objectives: The aim of this study was to estimate VAE incidence, detection of antimicrobial resistance pattern’s which can help in the management and to follow the outcome of the patients having VAE. Materials and methods: This was a retrospective study done over a period of 24months from January 2018 to December 2019 in a tertiary care hospital in Bangalore. The study population included all the confirmed VAE patients. Their incidence of microbiological profile and the outcome was noted. Results: Out of 422 mechanical ventilated (MV) patients 71 patients developed VAE. In the 71 proved VAE cases 48 (67.6%) were male and23 (32.4%) were females. Out of 71 cases, the mean age who were having VAE falls in the range of 65- 75 yrs. The incidence of VAE is 16.82 and the incidence density:9.78 /1000 ventilator days. Klebsiella pneumonia (29.5%) was the common organism isolated among 71 cases. Conclusion: Utilizing the preventive, diagnostic, and treatment recommendations outlined should allow for improved outcomes for a common and serious medical complication seen in ICU mechanically ventilated patients.
Keywords: Ventilator-associated event, Ventilator-associated event pneumonia, Mechanical ventilation
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Junior Infection Control Officer, Department of Pathology, Sagar Hospital, Bangalore, Karnataka, India. Email: |
Shashikala N, Manasa S, Hedge D, Vikram V, Mahendra KP. Incidence, microbiological profile and outcome of ventilator-associated events in a tertiary care hospital in Bangalore, Karnataka, India. Trop J Pathol Microbiol. 2020;6(2):130-138. Available From https://pathology.medresearch.in/index.php/jopm/article/view/420 |


 ©
©