Detection of dengue virus in Eastern India
Mukherji T.1, Mukherji MB.2*
DOI: https://doi.org/10.17511/jopm.2020.i01.12
1 Tamasi Mukherji, Assistant Professor, Department of Microbiology, KPC Medical College and Hospital, Kolkata, West Bengal, India. https://orcid.org/0000-0003-2119-2315
2* Mayur Bahan Mukherji, Associate Professor, Department of Medicine, KPC Medical College and Hospital, Kolkata, West Bengal, India.
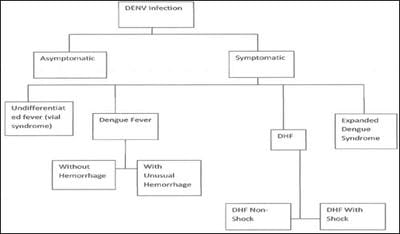
Background: Dengue fever is a mosquito-borne tropical disease caused by the dengue virus. Symptoms typically begin three to fourteen days after infection. The virus has four serotypes; infection with one type usually gives lifelong immunity to that type, but only short-term immunity to the others. Subsequent infection with different dengue virus increases the risk of severe complications. Methods: There are three methods followed in particular to detect the dengue virus: (A) Dengue NS1 AG Microlisa is designed for in-vitro qualitative detection of Dengue NS1 antigen in human serum or plasma and is used as a screening test for testing of collected blood samples suspected for DENGUE. This method detects all four subtypes; DEN1, DEN2, DEN3 & DEN4 of Dengue Virus. Results: Among 1860 dengue samp1es, 420 sâmp1es were found to be NS1Ag positive 160 samples were found to be IgM positive and 24 samples were found to be IgG positive. Rest Samples negative for all three parameters. Conclusion: Dengue is a mosquito borne viral infection causing a severe flu like illness and sometimes causing a potentially lethal complication called severe dengue. The incidence of dengue has increased 30 fold over the last 50 years. Up to 50-100 million infections are now estimated to occur annually in over 100 endemic countries, putting almost half of the world’s population at risk. If sever dengue fever can damage the lungs, liver or heart or multiorgan failure.
Keywords: Dengue virus, NS1 Antigen, IgM antibody, IgG antibody, Dengue hemorrhagic fever, Dengue shock syndrome, ELISA
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Medicine, KPC Medical College and Hospital, Kolkata, West Bengal, India. Email: |
Mukherji T, Mukherji MB. Detection of dengue virus in Eastern India. Trop J Pathol Microbiol. 2020;6(1):76-82. Available From https://pathology.medresearch.in/index.php/jopm/article/view/417 |


 ©
©