Scrub typhus – atypical presentations: a case series from the state of Sikkim
Mohanty A.1*, Kabi A.2, Kumar Jha M.3, Rekha S.4, K R A.5, Gupta P.6
DOI: https://doi.org/10.17511/jopm.2019.i08.10
1* Aroop Mohanty, Senior Resident, Department of Microbiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India.
2 Ankita Kabi, Assistant Professor, Department of Trauma and Emergency Medicine, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India.
3 Mithilesh Kumar Jha, Senior Resident, Department of Microbiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India.
4 Sasi Rekha, Junior Resident, Department of Microbiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India.
5 Anusha K R, Junior Resident, Department of Microbiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India.
6 Pratima Gupta, Professor and Head, Department of Microbiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India.
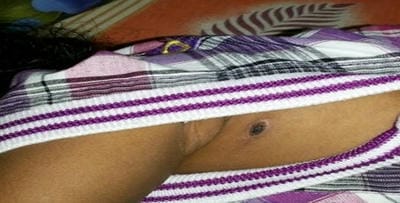
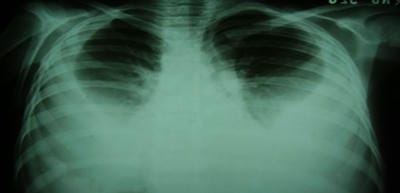
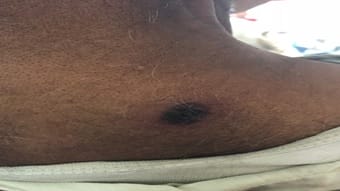
Scrub typhus is caused by Orientia tsutsugamushi and is transmitted to humans by an arthropod vector of the Thrombiculidae family. It is one of the most common re-emerging ricketssial infection in India and other South east Asian countries. Nearly a billion people are at risk with at least a million cases being reported from this region every year. It is distributed in the tsutsugamushi triangle which is distributed over a wide area of 13 million km2. Eschar is the characteristic lesion that starts as a vesicular lesion at the site of mite feeding. It is present in about 40% of cases. It progresses to an ulcer with black necrotic center and an erythematous border along with regional lymphadenopathy. It may affect the central nervous system, cardiovascular system, renal and gastrointestinal system. Here we report three cases which depict the atypical presentations of this disease. It is an eye-opener for clinicians to keep this as a provisional diagnosis in patients who present with fever of unknown origin.
Keywords: Scrub typhus
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Senior Resident, Department of Microbiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. Email: |
Mohanty A, Kabi A, Jha MK, Rekha S, Anusha K R, Gupta P. Scrub typhus – atypical presentations: a case series from the state of Sikkim. Trop J Pathol Microbiol. 2019;5(8):568-573. Available From https://pathology.medresearch.in/index.php/jopm/article/view/311 |


 ©
©