Expression of vascular endothelial growth factor (VEGF) in IgA nephropathy
Gomathi R.1, Adayalam C.2*
DOI: https://doi.org/10.17511/jopm.2019.i08.13
1 Gomathi R., Shri Sathya Sai Medical College, Sri Balaji Vidhyapeeth University, Chennai, Tamil Nadu, India.
2* Chandana Adayalam, Shri Sathya Sai Medical College, Sri Balaji Vidhyapeeth University, Chennai, Tamil Nadu, India.
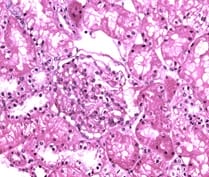
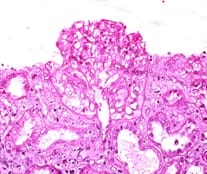
Background: Vascular endothelial growth factor (VEGF) is an important mediator involved in maintaining normal kidney function. Loss of normal or controlled secretion of VEGF following damage to the visceral epithelial cells could lead to alterations in endothelial cell function.This study was carried out to evaluate expression of VEGF in IgA nephropathy in order to look for potential diagnostic markers and predictive markers for progression towards CKD. Methods: This retrospective record based cross sectional study was carried out on a total of 16 whole renal cell biopsy specimens. Following the diagnosis of IgA nephropathy, immunohistochemical techniques were used to evaluate the VEGF expression. The expression of VEGF was graded and evaluated specific for the type of IgA nephropathy. Results: VEGF expression was positive in 6 specimens (37.5%). The type of glomerular involvement showing VEGF positivity was mesangial proliferative glomerulonephritis, minimal change disease (12.5%), post infectious glomerulonephritis (6.25%) and focal-segmental glomerulosclerosis (6.25%). All the specimens which showed VEGF positivity were showing well-defined areas with positive cells (25-50%). Conclusion: Considering the high magnitude of VEGF expression, the present study has potentiated the need for evaluating the statistical significance in attributing VEGF to IgA nephropathy in a larger volume of specimens.
Keywords: IgA nephropathy, Immunohistochemistry, Minimal Change disease, Vascular Endothelial growth factor
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Shri Sathya Sai Medical College, Sri Balaji Vidhyapeeth University, Chennai, Tamil Nadu, India. Email: |
Gomathi R, Adayalam C. Expression of vascular endothelial growth factor (VEGF) in IgA nephropathy. Trop J Pathol Microbiol. 2019;5(8):585-590. Available From https://pathology.medresearch.in/index.php/jopm/article/view/307 |


 ©
©